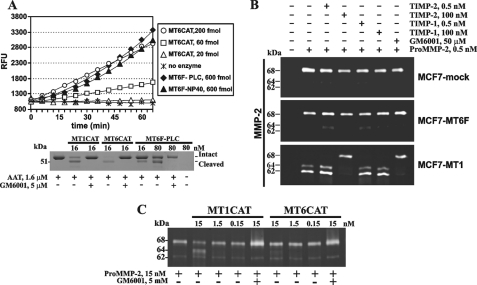
FIGURE 5.
Proteolytic activity of MT6-MMP. A, top, the proteolytic activity of MT6CAT (20–200 fmol), MT6F-PLC, and MT6F-Nonidet P-40 (600 fmol each) was measured using Mca-PLGL-Dpa-AR-NH2 as a substrate (5 μm). Bottom, MT6F-PLC is catalytically active and cleaves AAT. AAT (1.6 μm) was co-incubated with the indicated concentration of MT1CAT, MT6CAT, and MT6F-PLC. Where indicated, GM6001 (5 μm) was added to the reactions. The reactions were analyzed by SDS-PAGE, followed by Coomassie staining. RFU, relative fluorescence unit. B, gelatin zymography of the condition medium aliquots from MCF7-mock, MCF7-MT1, and MCF7-MT6F cells. Where indicated, pro-MMP-2 (0.5 nm), TIMP-1 (0.5–100 nm), TIMP-2 (0.5–100 nm), and GM6001 (50 μm) were added to the cells. C, the individual catalytic domain of MT6-MMP exhibits a limited ability to activate pro-MMP-2 in the cell-free system. The indicated concentrations of MT1CAT and MT6CAT were co-incubated with pro-MMP-2 (15 nm). The samples were then analyzed by gelatin zymography. Where indicated, GM6001 (5 μm) was added to the reactions to block MMP activity.