FIGURE 2.
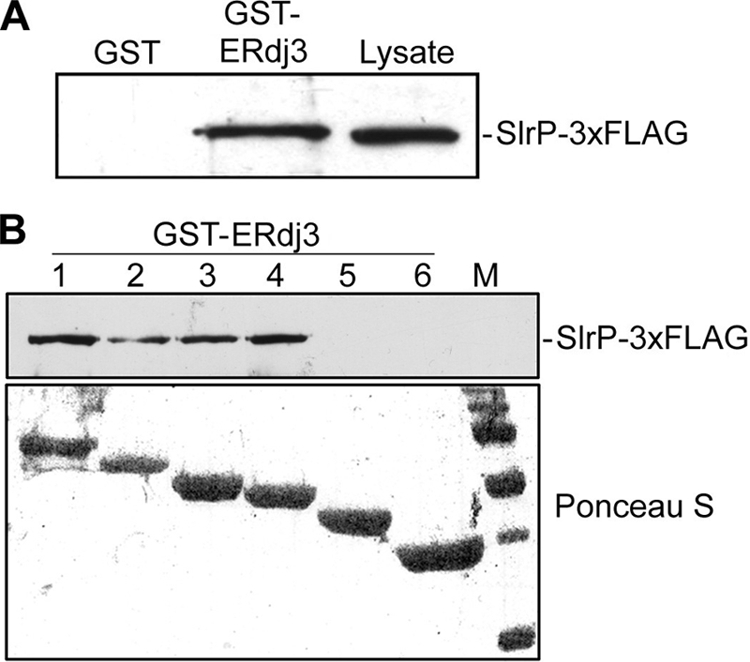
Interaction of ERdj3 and SlrP in vitro. A, in vitro binding of SlrP-3×FLAG to GST-ERdj3. Expression of GST and GST-ERdj3 proteins was induced with isopropyl-1-thio-β-d-galactopyranoside, and proteins were isolated from bacterial lysates by affinity chromatography with glutathione-agarose beads. 4 μg of each GST protein were incubated with lysates from 109 cfu of S. enterica serovar Typhimurium strain SV5193 (14028 slrP::3×FLAG) prepared in Nonidet P-40 lysis buffer. After washing, proteins eluted in sample buffer were resolved in 12% SDS-PAGE, blotted on nitrocellulose filters, and developed with monoclonal anti-FLAG antibody. Lysate from 108 cfu of the same strain is also included for reference. B, domain II of ERdj3 is essential for interaction with SlrP. Deleted derivatives of ERdj3 1–6 (see Fig. 1A) were expressed in fusion with GST and used for binding experiments with lysates from 109 cfu of Salmonella strain SV5193 as in A. M, molecular mass markers: visible bands correspond to 150, 100, 75, 50, 37, and 25 kDa.
