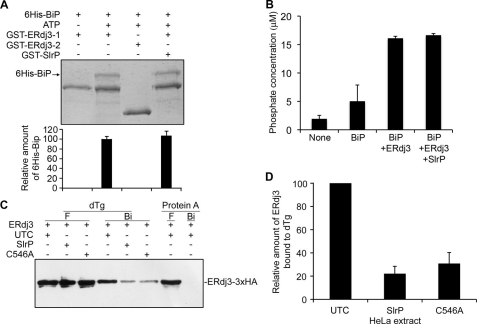
FIGURE 5.
Effect of SlrP on ERdj3 function. A, binding of ERdj3 to BiP. 50 μg of GST-ERdj3–1 (amino acids 23–358) or GST-ERdj3–3 (amino acids 129–358) immobilized in glutathione-agarose beads were incubated with 50 μg of His6-BiP in the presence or absence of 1 mm ATP and 50 μg of GST-SlrP as indicated. After incubating at 4 °C for 2 h, the beads were washed several times with binding buffer, and bound proteins were released by boiling for 5 min in sample buffer and subjected to 10% SDS-PAGE. Proteins were detected by Coomassie Blue staining. One stained gel is shown together with a graphical representation (below) of means ± S.D., from two independent experiments, of the quantification of bands of His6-Bip. B, stimulation of BiP ATPase activity by ERdj3. ATPase assays were performed with either no additions (None), 1 μg of His6-BiP plus 2.5 μg of GST (BiP), 1 μg of His6-BiP plus 2.5 μg of GST-ERdj3–1 plus 2.5 μg of GST (BiP+ERdj3), or 1 μg of His6-BiP plus 2.5 μg of GST-ERdj3–1 plus 2.5 μg GST-SlrP (BiP+ERdj3+SlrP). The amount of released phosphate was determined after incubation at 25 °C for 75 min with a non-radioactive procedure (see “Experimental Procedures”). Values are the mean of three separate experiments with an error bar representing S.D. C, binding of ERdj3 to dTg. Lysates from HeLa cells transiently expressing ERdj3–3×HA were mixed with lysates from untransfected cells (UTC) or with lysates transfected with SlrP or SlrPCys546Ala (C546A), as indicated, and then incubated with dTg or native protein A immobilized on agarose beads. After several washes with Nonidet P-40 buffer, proteins bound to the beads (Bi) were released by boiling in sample buffer, separated by using 10% SDS-PAGE and immunoblotted with anti-HA monoclonal antibodies. Part of the initial flow-through (F) obtained after the incubations was also included. D, image quantification of the relative amount of ERdj3 bound to immobilized dTg. The results are the mean ± S.D. of three independent experiments. The highest level was set to 100 for each experiment.