Abstract
Large-cell neuroendocrine carcinoma of the colon is a rare entity with a prognosis that is usually poor due to the high likelihood of early metastasis. A 61-year-old man had surgery for colon cancer of the transverse colon and cecum. Microscopic examination of the tumor showed that the location was the proximal transverse colon, with small nests containing rosettes and palisading patterns of large tumor cells with faintly granular cytoplasm. The immunohistochemistry was positive for synaptophysin and chromogranins. The tumors were diagnosed as a large-cell neuroendocrine carcinoma of the colon. In addition, the tumor of the cecum showed microscopic findings consistent with a well-differentiated adenocarcinoma. The immunohistochemical panel showed that the tumor was negative for neuroendocrine markers. There were no clinical findings suggestive of hormone hypersecretion. Cancer metastasis was found in the peritoneum section of the small bowel. Postoperative chemotherapy was applied. The patient was alive with good performance after, and there was no sign of tumor progression. This is the first case of a synchronous large-cell neuroendocrine carcinoma and adenocarcinoma of the colon. The patient was treated successfully with debulking surgery and systemic chemotherapy.
Keywords: Neuroendocrine carcinoma, Colonic neoplasms, Multiple primary neoplasms, Chemotherapy
INTRODUCTION
Neuroendocrine carcinomas are malignant tumors that show histopathological and immunohistochemical evidence of neuroendocrine differentiation. Neuroendocrine tumors can develop at many different sites of the body, but the colon is a very rare site.1 Most neuroendocrine tumors are carcinoids, and they have a better prognosis than conventional adenocarcinomas. On the other hand, large-cell neuroendocrine carcinomas (LCNEC) are usually aggressive and have the poor prognosis.2 There are few reports on large-cell colorectal neuroendocrine carcinomas. Here, we report the first case of LCNEC of the transverse colon that coexisted with an adenocarcinoma of the cecum.
CASE REPORT
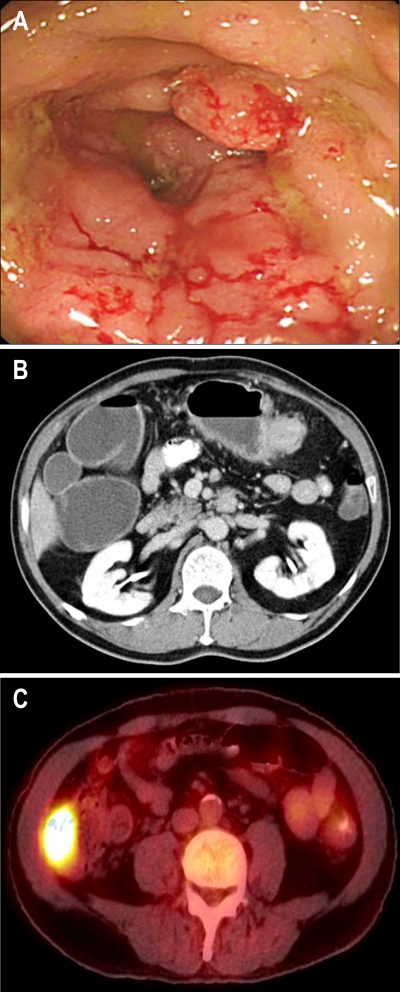
A 61-year-old man was admitted because of abdominal discomfort. The past history was unremarkable. Colonoscopy revealed an ulcerative infiltrating tumor involving the whole circumference of the colon in the proximal transverse portion; a biopsy was performed (Fig. 1A). We could not perform colonoscopic examination above the transverse colon due to luminal narrowing. The biopsy specimens were evaluated by traditional light microscopy and immunohistochemical staining. The pathology was consistent with a LCNEC. The abdominal computed tomography showed a 5 cm tumor in the transverse colon, enlargement of multiple regional lymph nodes and a 5 cm tumor in the ascending colon (Fig. 1B). Positron emission tomography revealed abnormal fluorodeoxyglucose uptake in the transverse colon and the ascending colon (Fig. 1C). There were no distant metastases. The carcinoembryonic antigen and carbohydrate antigen 19-9 were within normal limits. The patient was then referred to our department of surgery.
Fig. 1.
(A) Colonoscopy showed a bulky mass encircling the transverse colon and luminal narrowing. (B) CT showed a poorly enhancing mass in the proximal transverse colon with pericolic infiltration and regional lymph node enlargement. (C) Positron-emission tomography revealed abnormal fluorodeoxyglucose uptake in the cecum.
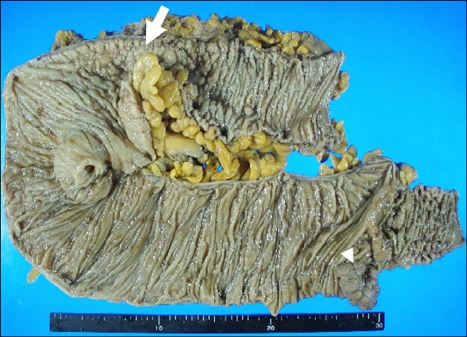
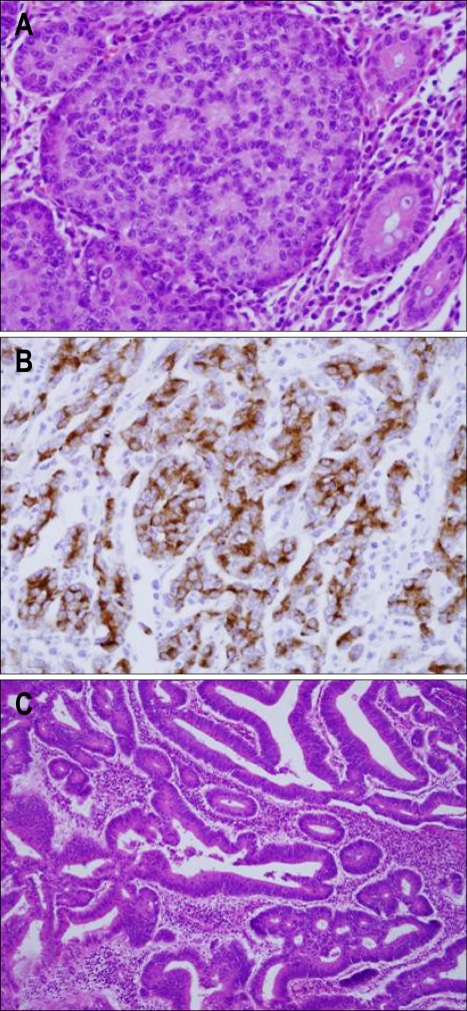
A right hemicolectomy was performed and two tumors were identified (Fig. 2). The first resected tumor was located in the proximal transverse colon and formed an ulcerative infiltrating mass, measuring 6.0×4.0 cm. This tumor invaded the pericolic adipose tissue. The resection margin was free from tumor. Cancer metastasis was found in 7 out of the 20 dissected regional lymph nodes. In addition, we found a metastatic mass in the peritoneum portion of the small bowel, and a biopsy was performed. The cross-section of the primary mass was a white-gray color, solid, poorly demarcated, and had focal necrosis. On microscopic examination of the primary mass, a metastatic mass, and lymph nodes, small nests, rosette like and palisading patterns of large tumor cells with faintly granular cytoplasm were present. There were numerous mitotic figures. An immunohistochemical panel was performed, and the tumor was found to be positive for chromogranins and synaptophysin. The CD56 was negative. Therefore, our diagnosis of the tumor was a large-cell neuroendocrine carcinoma (Fig. 3A, B).
Fig. 2.
Macroscopically, an ill-defined, ulcerative, and infiltrating mass was seen on the cut surface of the transverse colon (arrow), and a well demarcated, polypoid tumor was seen in the cecum (arrowhead).
Fig. 3.
Histological and immunohistochemical examination. (A) Rosettes in the solid tumor nests and large polygonal cells (H&E stain, ×400). (B) Immunohistochemical staining revealed positive findings for chromogranin (Immunohistochemical stain for chromogranin, ×400). (C) Pathological examination of the mass in the cecum revealed an adenocarcinoma (H&E stain, ×100).
A second resected tumor was located in the cecum. This was a polypoid tumor, measuring 5.0×4.0 cm. All sampled lymph nodes and surgical cut ends were free of tumor involvement. The depth of invasion was submucosa. The microscopic examination revealed a well differentiated adenocarcinoma (Fig. 3C). On the immunohistochemical staining panel, the tumor was negative for neuroendocrine markers (chromogranins, synaptophysin and CD56). Thus, we concluded that the patient had simultaneous development of a primary colon cancer and a primary LCNEC in the colon. The patient received postoperative chemotherapy with oxaliplation, 5-fluorouracil and leucovorin. Five cycles of chemotherapy were performed. The patient is still alive with good performance and no sign of tumor progression or distant metastasis 6 months after the treatment.
DISCUSSION
This case has two aspects of clinical interest. First, no previous case has been reported with synchronous adenocarcinoma and LCNEC in the colon. Second, there are no clear guidelines for appropriate treatment and follow up of LCNEC with a synchronous secondary malignancy; therefore, our experience may help in the consideration of treatment for similar patients.
LCNECs are rare; they account for <1% of colorectal cancers, and almost all have metastasized at the time of diagnosis, and therefore they have a poor prognosis.3,4 LCNEC is a neuroendocrine tumor that has features in common with neuroendocrine-differentiated tumors as well as specific cytological features: large cell size, polygonal shape, low nuclear-cytoplasm ratio, a mitotic index in excess of 10/10 per high power field, occasionally prominent nucleoli, and necrosis.5 The tumors are argyrophilic and stain positive for immunohistochemical panels including synaptophysin, chromogranin and neuron specific enolase. In patients known to have neuroendocrine tumors, the possibility of a hypersecretion syndrome such as the paraneopalstic or carcinoid syndrome always has to be considered. In the report by Vilallonga et al.3, on neuroendocrine tumors, excluding carcinoid tumors, five out of 2,155 colorectal cancer patients were identified; all of the patients presented with a paraneoplastic or carcinoid syndrome. However, our patient had no such symptoms. However, we cannot rule out the possibility of secreted hormones in the plasma at very low concentrations not associated with a clinical syndrome, or if there were secreted peptides they were not associated with clinical effects.
The precise pathogenesis of secondary cancers associated with neuroendocrine carcinoma remains unclear. Some reports have suggested that neuroendocrine tumors occur as secondary primary carcinoma. According to one previous study, gastrin and cholecystokinin were associated with neuroendocrine carcinoma; this resulted in tissue growth in the gastrointestinal tract and carcinogenesis leading to colorectal and gastric cancer.6 In addition, Prommegger et al.7 reported that neuroendocrine tumors are associated with a high risk of a secondary gastrointestinal malignancy. They studied 96 patients with neuroendocrine tumors and found that 14 patients had a neuroendocrine tumor and a second primary malignancy. Five patients had a synchronous second primary malignancy. The locations were two colon cancers with one double colon cancer, one gastric cancer, one bladder cancer, and one ovarian cancer. In a recent study, Kato et al.8 reported a CK 20 positive LCNEC of the colon, and suggested a possible link between colorectal neuroendocrine carcinoma and adenocarcinoma. These reports are consistent with the findings in our case where the patient had an advanced stage of neuroendocrine carcinoma in the transverse colon and a simultaneous early stage adenocarcinoma in the cecum. It is possible that the advanced stage neuroendocrine carcinoma was associated with the development of the adenocarcinoma of the colon. However, further confirmation is needed to support this.
Generally, surgical intervention for metastatic neuroendocrine tumors is not performed. If the neuroendocrine carcinoma is completely resected, the 5-year survival rate has been reported to be 61%. However, most patients are not candidates for curative resection. When complete resection is not possible, a debulking procedure should be performed in combination with cytoreductive therapy or systemic chemotherapy.9 The efficacy of systemic chemotherapy is highest in patients with poorly differentiated neuroendocrine tumors with a combination of cisplatin and etoposide.10
In our patient, a debulking procedure was performed. We could not completely resect the small bowel metastases. The patient received systemic chemotherapy with oxaliplatin, 5-fluorouracil and leucovorin. This regimen is usually used for advanced colon cancer. This was chosen instead of cisplatin and etoposide due to the coexistence of the adenocarcinoma. The oxaliplatin was expected to treat the LCNEC. Oxaliplatin is in the diaminocyclohexane platinum group; it inhibits DNA synthesis in cancer cells, and has the same pathophysiology as other platinum anticancer drugs such as cisplatin and carboplatin.11 The oxaliplatin, 5-fluorouracil and leucovorin based systemic chemotherapy was chosen for the potential anticancer effects in both the LCNEC and advanced adenocarcinoma. Two cycles of systemic chemotherapy were provided. The patient is still alive with a good performance status and no sign of tumor progression.
In conclusion, we report a LCNEC of the colon that coexisted with an adenocarcinoma, suggesting the possible association between colorectal neuroendocrine carcinoma and adenocarcinoma. Further investigations are required to determine the pathogenesis of these synchronous tumors.
References
- 1.Kloppel G, Perren A, Heitz PU. The gastroenteropancreatic neuroendocrine cell system and its tumors: the WHO classification. Ann N Y Acad Sci. 2004;1014:13–27. doi: 10.1196/annals.1294.002. [DOI] [PubMed] [Google Scholar]
- 2.Emory RE, Jr, Emory TS, Goellner JR, Grant CS, Nagorney DM. Neuroendocrine ampullary tumors: spectrum of disease including the first report of a neuroendocrine carcinoma of non-small cell type. Surgery. 1994;115:762–766. [PubMed] [Google Scholar]
- 3.Vilallonga R, Espin Basany E, Lopez Cano M, Landolfi S, Armengol Carrasco M. Neuroendocrine carcinomas of the colon and rectum: a unit's experience over six years. Rev Esp Enferm Dig. 2008;100:11–16. doi: 10.4321/s1130-01082008000100003. [DOI] [PubMed] [Google Scholar]
- 4.Grabowski P, Schonfelder J, Ahnert-Hilger G, et al. Expression of neuroendocrine markers: a signature of human undifferentiated carcinoma of the colon and rectum. Virchows Arch. 2002;441:256–263. doi: 10.1007/s00428-002-0650-9. [DOI] [PubMed] [Google Scholar]
- 5.Krivak TC, McBroom JW, Sundborg MJ, Crothers B, Parker MF. Large cell neuroendocrine cervical carcinoma: a report of two cases and review of the literature. Gynecol Oncol. 2001;82:187–191. doi: 10.1006/gyno.2001.6254. [DOI] [PubMed] [Google Scholar]
- 6.Reim D, Weirich G, Neu B, Bajbouj M, Brucher BL. Synchronous adenocarcinoma of the lung and neuroendocrine carcinoma of the ileum. Int J Colorectal Dis. 2008;23:325–327. doi: 10.1007/s00384-007-0412-x. [DOI] [PubMed] [Google Scholar]
- 7.Prommegger R, Ensinger C, Steiner P, Sauper T, Profanter C, Margreiter R. Neuroendocrine tumors and second primary malignancy: a relationship with clinical impact? Anticancer Res. 2004;24:1049–1051. [PubMed] [Google Scholar]
- 8.Kato T, Terashima T, Tomida S, et al. Cytokeratin 20-positive large cell neuroendocrine carcinoma of the colon. Pathol Int. 2005;55:524–529. doi: 10.1111/j.1440-1827.2005.01864.x. [DOI] [PubMed] [Google Scholar]
- 9.Arnold R. Endocrine tumours of the gastrointestinal tract. Introduction: definition, historical aspects, classification, staging, prognosis and therapeutic options. Best Pract Res Clin Gastroenterol. 2005;19:491–505. doi: 10.1016/j.bpg.2005.03.006. [DOI] [PubMed] [Google Scholar]
- 10.Plockinger U, Wiedenmann B. Endocrine tumours of the gastrointestinal tract: management of metastatic endocrine tumours. Best Pract Res Clin Gastroenterol. 2005;19:553–576. doi: 10.1016/j.bpg.2005.02.013. [DOI] [PubMed] [Google Scholar]
- 11.Mamenta EL, Poma EE, Kaufmann WK, Delmastro DA, Grady HL, Chaney SG. Enhanced replicative bypass of platinum-DNA adducts in cisplatin-resistant human ovarian carcinoma cell lines. Cancer Res. 1994;54:3500–3505. [PubMed] [Google Scholar]