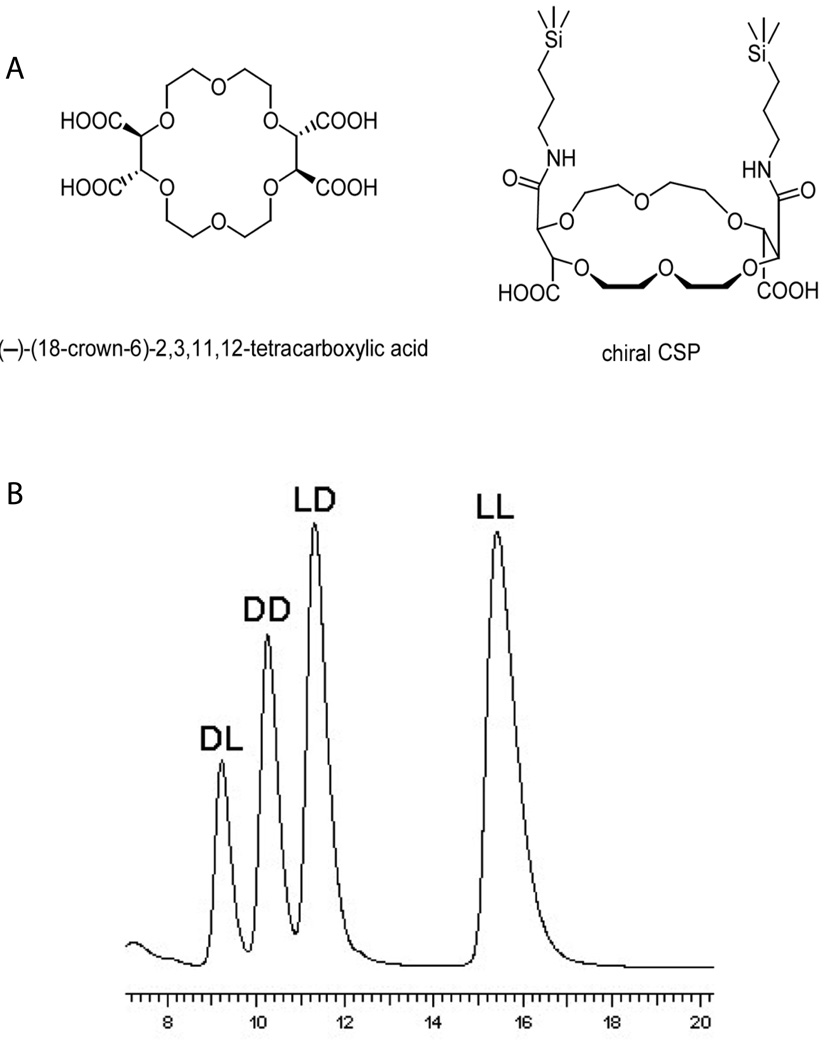
Fig. 4.
(A) Crown ether (−)-(18-Crown-6)-tetracarboxylic acid. (B) HPLC separation of Ala-Phe stereoisomers on a (−)-(18-Crown-6)-tetracarboxylic acid-based column. Interaction between protonated amine groups in peptides and oxygen atoms lining the inner surface of the crown ether molecule is the basis of chiral selectivity. 10 mM sulfuric acid in 70% methanol-water was used as mobile phase to facilitate protonation of amine groups. Adapted with permission from Wiley-VCH Verlag GmbH & Co. KGaA [62].