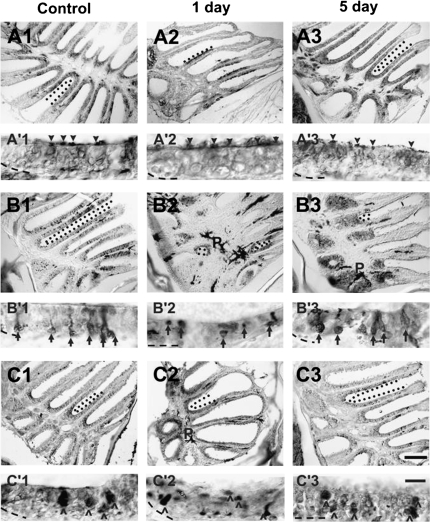
Figure 5.
Antibodies were used to characterize the effects of chemical ablation on specific cell types of the zebrafish OE. (A) Anti-Gα s/olf in control epithelia (A1) revealed immunoreactive profiles throughout the sensory region of the rosette. Dendritic knobs and cilia were distinctly labeled (A′1, arrowheads), and there was staining found diffusely through the depth of the epithelium except the basal region. One day after Triton X-100 application, there were fewer Gα s/olf -positive areas in the epithelium (A2), but where labeling occurred, the dendritic knobs and cilia of ciliated neurons were marked (A′2) with only faint labeling of the epithelium underneath. Five days after chemical lesion (A3), however, α-Gα s/olf staining had returned to normal levels and pattern (A′3). (B) Anti-calretinin labeling in control olfactory organs identified discrete cell bodies and their dendritic processes (B′1, arrows) scattered throughout the OE (B1). Immunoreactivity to this cell marker was greatly diminished 1 day following Triton X-100 treatment (B2), and those cells labeled appeared to be without dendritic knobs (B′2). Labeling to anti-calretinin was increased by 5 days after treatment (B3), and the bodies that were labeled also had obvious processes (B′3). (C) Anti-S100 labeled large round cells (^ in C′1), the crypt neurons, scattered around the sensory regions in the OE of control rosettes (C1). S100-immunoreactivity showed an overall decrease 1 day after treatment (C2), and the bodies were smaller and flattened compared with control (C′2). Immunoreactivity recovered to near control levels (C3) and morphology (C′3) 5 days after lesioning. Demarcated with dotted lines are examples of areas where labeling was present within the sensory epithelium. p = pigment of the lamina propria in the central raphe and is not indicative of antibody labeling. Basement membrane is marked with a dashed line. Scale bar = 100 μm for A1–3, B1–3, C1–3 and 10 μm for A′1–3, B′1–3, C′1–3.