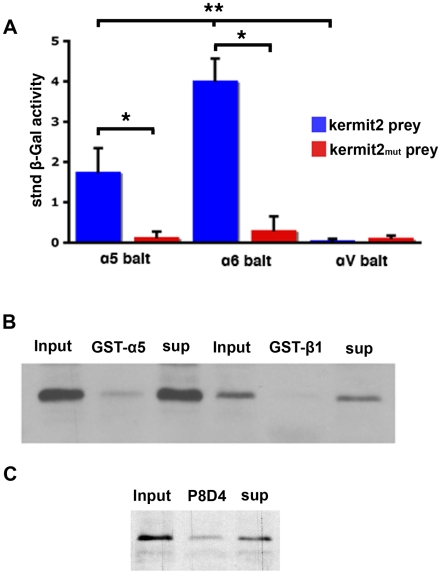
Figure 1. Kermit2 interacts with the cytoplasmic domain of the α5 and α6 integrin subunits.
(A) Yeast two two-hybrid assays were conducted using α5, α6 and αV cytoplasmic domains as bait in combination with kermit2 or kermit2mut as prey. The data is presented as average normalized β-galactosidase activity (±SD). The interaction of kermit2 with α5 and α6 is abolished by the AEEL mutation in kermit2mut (* P<0.002). The αV subunit does not interact with kermit2 or kermit2mut (** indicates P<0.001 between α5 or α6 and αV bait constructs). (B) GST pulldowns. HA tagged Kermit2 is detected in lysates (lane 1 input), and is pulled down with a GST-α5 fusion construct (lane 2 GST-α5). Most of the kermit2 remains in the supernatant (lane 3 sup). A control GST-β1 construct (lane 5 GST-β1) does not pull Kermit2 from lysates (lane 4 input, lane 6 sup). (C) Coimmunoprecipitation of kermit2 with α5β1 integrin. HA tagged kermit2 is detected in lysates (lane 1 input). Kermit2 is found in association with immunoprecipitated α5β1 (lane 2 P8D4), while a significant portion of kermit2 remains in the supernatant (sup).