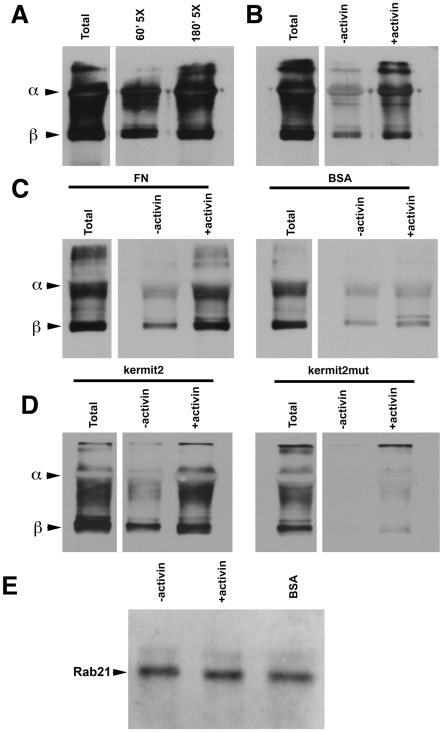
Figure 9. Endocytosis of α5β1 integrin.
Cell surface α5β1 integrin was labeled with cleavable biotin and endocytosed integrin was immunoprecipitated and integrin subunits detected with streptavidin HRP on non-reducing western blots. α and β subunits are indicated to the left of the panels. The non-reduced P8D4 IgG used for the immunoprecipitation runs at the same molecular weight as the α subunit partially masking the signal. In all panels the total lane represents five fold more cells than represented in other lanes and the samples were not surface stripped. (A) Time course of α5β1 integrin endocytosis following activin induction. Increasing amounts of α5β1 are found in the cytoplasm at 60 minutes and 180 minutes following activin treatment. (B) α5β1 integrin endocytosis is stimulated by activin induction. Panels A&B come from the same gel and are separated for clarity. (C) Cell adhesion stimulates α5β1 endocytosis. Activin-tretaed cells adherent on FN substrates exhibit an increased rate of endocytosis as compared to cells on a non-adherent (BSA) substrate. (D) Kermit2 regulates α5β1 endocytosis. Activin-treated kermit2mut expressing cells show reduced levels of α5β1 integrin endocytosis as compared to cells expressing kermit2. (E) Rab 21 coprecipitates with α5β1 integrin independent of mesoderm induction and adhesive substrate. Rab 21 was detected in α5β1 immunoprecipitates with A-14 antibody.