Table 1.
Rate constants for the cycloaddition of dibenzocyclooctynol 2 with nitrones 4 a–f.[a]
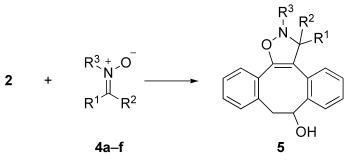 | ||||||
|---|---|---|---|---|---|---|
| 4 | R1 | R2 | R3 | k[b] [m−1 s−1] | k′ | Yield [%] |
| a | H | Ph | Me | 1.3×10−2 | 1 | 95 |
| b | H | CH2CH2Ph | Me | 3.2×10−2 | 3 | 80 |
| c | H | Ph | Ph | >0.2[c] | >17 | 89 |
| d | Me | CH2CH2CO2Et | Me | <1×10−3 | <0.1 | 33 |
| e | H | CO2Et | Me | 3.9 | 330 | 92 |
| f | H | C(O)NHBn | Me | 2.2 | 180 | 93 |
The nitrone substrates (except 4 d) were formed as pure Z isomers. Isoxazoline 5 was formed as a mixture of regio- and diastereoisomers. See the Supporting Information for reaction conditions.
Method A: The rate constant was determined by 1H NMR spectroscopy in CD3CN/D2O (3:1); [2 a]=18 mm, [4 a–f]=16.4 mm. Method B: The rate constant was determined18 by UV spectroscopy in CH3CN/H2O (3:1); [2 a]=0.33 mm, [4 a–f]=0.30 mm. These reactions were too fast for monitoring by NMR spectroscopy.
The reaction was too fast for accurate determination of the rate constant by NMR spectroscopy. Determination by UV spectroscopy was not possible owing to overlapping absorptions. Bn=benzyl.
