Abstract
OBJECTIVE
To determine if ProL1, a member of the opiorphin family of genes, can modulate erectile physiology, as it encodes a peptide which acts as a neutral endopeptidase inhibitor, other examples of which (Vcsa1, hSMR3A) modulate erectile physiology.
MATERIALS AND METHODS
We cloned members of the opiorphin family of genes into the same mammalian expression backbone (pVAX); 100 μg of these plasmids (pVAX-Vcsa1, -hSMR3A, -hSMR3B and -ProL1) were injected intracorporally into retired breeder rats and the affect on erectile physiology assessed visually, by histology and by measuring the intracavernous pressure (ICP) and blood pressure (BP). As a positive control, rats were treated with pVAX-hSlo (expressing the MaxiK potassium channel) and as a negative control the empty backbone plasmid was injected (pVAX). We also compared the level of expression of ProL1 in corporal tissue of patients not reporting erectile dysfunction (ED), ED associated with diabetes and ED not caused by diabetes.
RESULTS
Gene transfer of plasmids expressing all members of the opiorphin family had a similar and significant effect on erectile physiology. At the concentration used in these experiments (100 μg) they resulted in higher resting ICP, and histological and visual analysis showed evidence of a priapiclike condition. After electrostimulation of the cavernous nerve, rats had significantly better ICP/BP than the negative control (pVAX). Gene transfer of pVAX-hSlo increased the ICP/BP ratio to a similar extent to the opiorphin homologues, but with no evidence for a priapic-like condition. Corpora cavernosa tissue samples obtained from men with ED, regardless of underlying causes, had significant down-regulation of both hSMR3A and ProL1.
CONCLUSION
All members of the human opiorphin family of genes can potentially modulate erectile physiology. Both hSMR3 and ProL1 are down-regulated in the corpora of men with ED, and therefore both genes can potentially act as markers of ED.
Keywords: Vcsa1, hSMR3, ProL1, opiorphin, erectile dysfunction, priapism
INTRODUCTION
We reported recently that the gene hSMR3A is down-regulated in patients with erectile dysfunction (ED) [1]. Gene transfer of plasmids expressing hSMR3A into the corporal tissue of ageing rats improved erectile function at lower doses and caused priapism at higher doses, suggesting a direct involvement of this gene in erectile physiology. hSMR3A belongs to a family of genes which include Vcsa1 (in the rat) and ProL1 (in humans), which encode the pentapeptides sialorphin and opiorphin, respectively (Table 1). hSMR3A has a close homologue called hSMR3B, which does not affect the region that encodes the hypothetical pentapetide product (hSMR3).
TABLE 1.
Pentapeptides encoded by the opiorphin family of genes
| Gene | Pentapetide | Species | Amino-acid sequence |
|---|---|---|---|
| Vcsa1 | Sialorphin | Rat | QHNPR |
| ProL1 | Opiorphin | Human | QRFSR |
| hSMR3A/B | hSMR3 | Human | QRGPR (hypothetical peptide) |
| Consensus | QrpR | ||
| Qr.pR |
The expression of this family of genes appears to be restricted to relatively few tissues. They were first isolated in the submanibular gland, but subsequently have been shown to be expressed in corporal tissue [2,3]. There is accumulating evidence to suggest the opiorphins are important in mammalian sexual function. There is a marked genderspecific expression, with male rats expressing 1000 times more of the Vcsa1 transcript in the submandibular glands than female rats [4]. Male rats injected with sialorphin 45 min before being paired with sexually receptive females had a dose-dependent ability to modulate, at doses related to physiological circulating levels, the male rat mating pattern, exerting a dual facilitative or inhibitory dose-dependent effect on the sexual performance, while stimulating the apparent sexual arousal or motivation [5]. Expression of both Vcsa1 in rats and hSMR3 in humans is down-regulated in response to ED [1,3]. Intracorporal injection with a plasmid expressing hSMR3, Vcsa1 or the peptide, sialorphin, can increase erectile function (as determined by an increase in intracorporal pressure/blood pressure (ICP/BP) in retired breeder rats after electrostimulation) [1,3,6]. If large amounts of the plasmid encoding sialorphin or hSMR3 are injected into the rats they will cause a ‘priapic-like’ state in the rat penis.
Sialorphin acts as a potent neutral endopeptidase (NEP) inhibitor in rats, and human opiorphin has been shown to have similar activity [7,8]. NEPs are located at the surface of cells in neural and systemic tissues where they have important functions as ectoenzymes, catalysing the postsecretory processing or metabolism of several signalling peptides, and their inhibition can prolong the binding of peptide agonists to their receptors [9]. Signal peptides are involved in the receptor-dependent control of many physiological functions, e.g. central pain perception and depression, and peripheral inflammatory phenomena, arterial tone and mineral homeostasis. Particularly relevant to erectile physiology is the observation that inhibitors of NEP can cause relaxation of smooth muscle tissue [10,11]. Relaxation of corporal smooth muscle tissue is a prerequisite for erectile function in male animals [12,13]. Sialorphin appears to function in erectile physiology through its action as a NEP inhibitor, by modulating the tone of corporal smooth muscle tissue [6].
Opiorphin, encoded by ProL1, was recently shown to also act as a NEP inhibitor [8]. Therefore we determined if intracorporal gene transfer of plasmids expressing ProL1 (pVAX-ProL1) had a similar effect on erectile physiology as plasmids expressing Vcsa1 and hSMR3A. Because the gene encoding hSMR3 is down-regulated in human patients with ED we also determined if ProL1 could also act as a marker for ED.
MATERIALS AND METHODS
Experiments to determine the function of the opiorphin homologues in erectile physiology were carried out in 30 9- to 10-month-old (old) retired breeder male Sprague-Dawley rats (>500 g). Vectors/plasmids were microinjected into rat corporal tissue essentially as previously described [1,3]. Briefly, the rats were anaesthetized by an i.p. injection with pentobarbital sodium (35 mg/kg).The perineum was shaved using an electric shaver, cleaned with betadine solution (povidoneiodine, 10%) and 1-cm incision was made at the base of the corpora to expose the left crura. All micro-injections consisted of a single bolus of naked plasmid DNA into the left crura, using an insulin syringe (1/2 mL 28 G1/2). The total volume of all microinjections was 150 μL. The plasmid pVAX was obtained commercially (Invitrogen, Carlsbad, CA, USA). Construction of the plasmid pVAX-hSlo, pVAX-Vcsa1, and pVAX-hSMR3B was described previously [1,3,14]. To construct pVAX-ProL1, the pVAX vector and pCMV6-XL5-PROL1 vector (OriGene, Rockville, MD, USA) were digested with NotI/EcoRI. The NotI/EcoR1 fragment from pCMV6-XL5-PROL1 vector was then ligated to the NotI/EcoR1 site of pVAX1 vector. To construct pVAX-hSMR3A vector, pCMV6-XL5-SMR3A vector (OriGene) was digested with NotI/XbaI. The hSMR3A fragment was then cloned in the NotI/XbaI-digested pVAX vector. The plasmids pVAX1-hSMR3A and pVAX1-ProL1 were verified by DNA sequencing.
At 1 week after gene transfer the rats were anaesthetized by i.p. injection with pentobarbital sodium (35 mg/kg) and the ICP/BP determined before and during electrostimulation of the cavernous nerve, using the method described previously [1,3]. Briefly, an incision was made in the perineum, and a window was made in the ischiocavernosus muscle to expose the crus of the corpus cavernosum. The cavernous nerves were identified on the posterior lateral surface of the prostate gland. The cavernous nerve was electrostimulated directly using a delicate stainless-steel bipolar hook electrode attached to the multijointed clamp. Each probe was 0.2 mm in diameter; the two poles were separated by 1 mm. Monophasic rectangular pulses were delivered by the signal generator (custom-made and with built-in constant current amplifier; Grass Instruments, Astro-Medical Inc., Quincy, MA, USA). Stimulation parameters were 20 Hz, pulse width 0.22 ms and duration 1 min; current was applied at 0.75 and 4 mA. The changes in the ICP and BP were recorded at each level of neurostimulation, and compared with the negative control (pVAX, the empty parent vector). The mean (SD) ICP/BP and ANOVA were calculated for each of the treatment groups; differences from the negative control of P < 0.05 were considered statistically significant.
Human corporal tissue was procured from 10 patients during penile prosthetic implant surgery, according to protocols approved by the AECOM/Montefiore Hospital Internal Review Board. The conditions and ages of the patients were reported previously [1]. Briefly five patients had ED associated with diabetes, five had ED and were not diabetic and three control patients did not report ED.
For quantitative reverse-transcriptase (RT) PCR of ProL1 and hSMR3, total RNA was extracted from human frozen tissue, RNA prepared and quantitative RT-PCR performed as previously described [1]. The expression of transcripts was analysed using the comparative crossing threshold (Ct) method (also known as the 2–ΔΔCt method). The primers used for normalization (glyceraldehyde phosphate dehydrogenase) and hSMR3 were previously described. The primers used for ProL1 determination were: forward primer: 5′-GCTCTTATTTCATGTTTCACACCCAG-3′; reverse primer: 5′-TAACCCGGAAAGAGTCGAGGT-3′.
RESULTS
We previously reported that plasmids expressing Vcsa1 and hSMR3A result in improved erectile function at low doses and also cause priapism at higher doses [1,3]. We also reported that gene transfer of pVAX-hSlo improves erectile function with no evidence of priapism, even at doses of up to 1000 μg [15]. We investigated if a plasmid expressing the gene encoding ProL1 or hSMR3B (a splice variant of hSMR3A, in which the region of the gene encoding the hypothetical pentapeptide is unaltered) would modulate erectile physiology in a similar manner to Vcsa1 and hSMR3A. At 1 week after intracorporal injection with the plasmids (pVAX, pVAX-hSlo, pVAX-hSMR3A, pVAX-hSMR3B, pVAX-ProL1 and pVAX-Vcsa1) the rats were examined visually for signs of priapism (postmortem, Table 2) and the basal ICP/BP was determined in the rats (Fig. 1).
TABLE 2.
Visual assessment of erectile function after intracorporal gene transfer of the empty vector plasmid or plasmids expressing opiorphin homologues (Vcsa1, hSMR3A/B and ProL1) or hSlo
| Erection at 0.75 mA |
Erection at 4 mA |
|||||||
|---|---|---|---|---|---|---|---|---|
| Plasmid | No. of rats | Priapism* | None | Partial | Full | None | Partial | Full |
| pVAX | 5 | 0 | 5 | 4 | 1 | |||
| pVAX-hSlo | 5 | 0 | 2 | 3 | 5 | |||
| pVAX-Vcsa1 | 5 | 4 | 1 | 4 | 2 | 3 | ||
| pVAX-hSMR3A | 5 | 2 | 4 | 1 | 1 | 4 | ||
| pVAX-hSMR3B | 5 | 3 | 5 | 1 | 4 | |||
| pVAX-ProL1 | 5 | 4 | 3 | 2 | 5 | |||
Visual evidence of priapism.
FIG. 1.
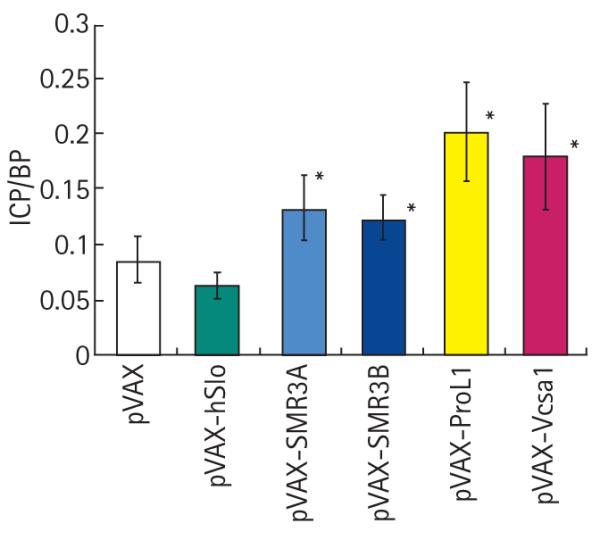
Comparison of the basal ICP at 1 week after intracorporal injection with 100 μg plasmids expressing hSlo, SMR3A, SMR3B, ProL1 or Vcsa1 with control (rats injected with the parent vector, pVAX). *P < 0.05 vs pVAX control.
Evidence for priapic episodes were either visually apparent penile tumescence or vasocongestion of corporal tissue confirmed by postmortem histological confirmation. None of the rats in the control groups (treated with pVAX and pVAX-hSlo) had any evidence of priapism (Table 2). In all groups treated with the opiorphin homologues, at least two of five rats had visual evidence of priapism. This was previously reported for Vcsa1 and hSMR3A [1,3], but not for ProL1 or hSMR3B.
The visual observation of priapism was supported by the basal ICP/BP. All the genes expressing opiorphin homologues (hSMR3A, hSMR3B, ProL1 and Vcsa1) caused a higher basal ICP than the empty vector controls. pVAX-hSlo, which we previously showed to be effective in improving ED in ageing rats, but has never caused priapism, gave no significant difference in resting basal ICP/BP from the empty vector controls.
After measuring the basal ICP/BP the cavernous nerve of the rats was electrostimulated with 0.75 and 4 mA, and the effect on erectile physiology monitored visually (Table 2) and by measuring the ICP/BP. In the negative control rats (treated with pVAX, the empty vector) none at the lower or higher level of stimulation had an observable full erection. With pVAX-hSlo, three of five at 0.75 mA and all five rats at 4mA had a full erection. The rats treated with plasmids expressing the opiorphin homologues had visually better erections than had control rats treated with pVAX after electrostimulation of the cavernous nerve.
The visual effects on erectile function were confirmed by measuring the ICP/BP response after electrostimulation (Fig. 2). The plasmid pVAX-hSlo (hMaxiK) is presently being evaluated in clinical trials for treating ED, and we have several reports showing that the plasmid can improve erectile function in rats. This plasmid, when transferred into rats, gave a better erectile function than the negative control (pVAX) at both stimulations. In addition, despite evidence of priapism, there was a significant increase in the ICP/BP ratio with plasmids expressing opiorphin and its homologues (pVAX-Vcsa1, pVAX-SMR3A, pVAX-SMR3B and pVAX-ProL1) after stimulation. Interestingly, although rats treated with pVAX-ProL1 developed an ICP/BP ratio normally indicative of a full erection, these rats were unable to maintain a full erection and were recorded visually as having only a partial erection.
FIG. 2.
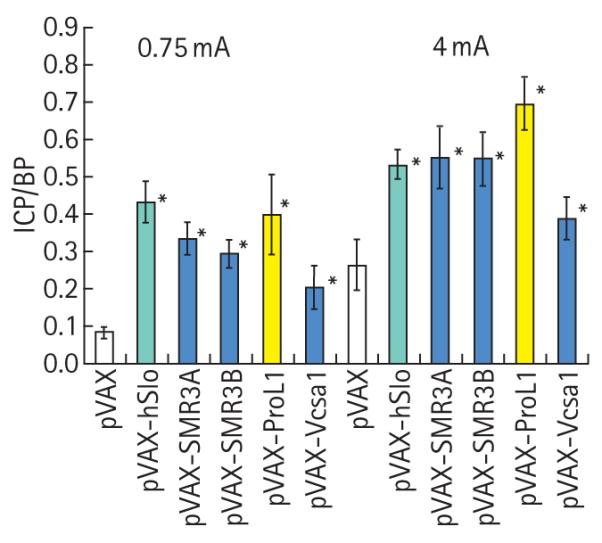
ICP/BP ratio after electrostimulation of the cavernous nerve in rats intracorporally injected with 100 μg of pVAX, pVAX-hSlo, pVAX-hSMR3A, pVAX-hSMR3B, pVAX-ProL1 and pVAX-Vcsa1. *Significantly different (P < 0.05) from negative control (pVAX).
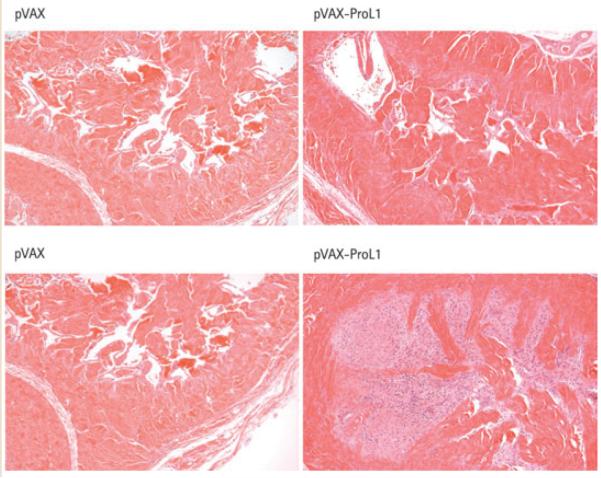
Visual postmortem examination of the corpora of rats that had intracorporal injection of pVAX-ProL1 had evidence of vascular congestion of the corporal tissue, suggesting they had had a ‘priapic-like’ episode. This was confirmed through histopathological examination of rats injected with 100 μg pVAX-ProL1. Trabecular interstitial oedema, blood clot formation within the cavernosa, and destruction of the endothelial lining was apparent with microscopy, suggesting that priapism had been severe in the 1 week between injection of the plasmid and termination of the experiment. As previously reported, this observation is similar to the effects of treating rats with pVAX-Vcsa1 or pVAX-hSMR3A [1,3]. By contrast, the plasmid pVAX-hSlo has never been observed to cause priapism after intracorporal injection, even at doses as high as 1000 μg [15]. In addition, rats treated with pVAX-ProL1 had marked hypertrophy and hyperplasia, and minimal inflammation in the region of the hyperplastic smooth muscle, which was interpreted to be the result of minimal degeneration of the hypertrophic cells. Neither negative control rats nor rats injected with the same dose of pVAX-hSlo showed any signs of priapism (Fig. 3).
FIG. 3.
Histological analysis of corporal tissue sections 1 week after intracorporal injection of 100 μg pVAX-ProL1 compared with control tissue (treated with pVAX). Two different sections of corpora from the same rat are shown.× 10.
We previously showed that hSMR3 expression is down-regulated in patients with ED. Using the same tissue samples described in [1] we analysed the expression of ProL1 and compared the changes in expression to those previously reported for hSMR3 [1]. In patients with ED there was a statistically significant nearly 10-fold decrease in the levels of ProL1 expression similar to the effect of ED on hSMR3 expression (Fig. 4). The effect on the gene expression was independent of whether the ED aetiology was diabetes- associated or not. These results suggest that all members of the opiorphin family of genes are potential markers of ED in patients.
FIG. 4.
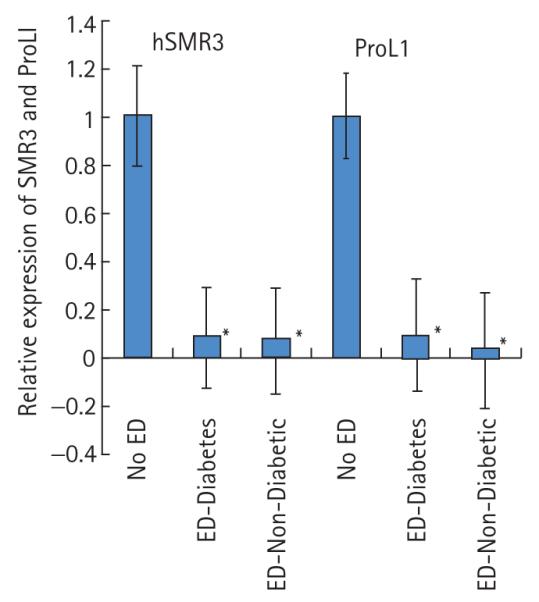
Comparison of the expression of SMR3 and ProL1 in patients without ED or with ED associated with a diabetic or non-diabetic aetiology.
DISCUSSION
The major conclusion of this study is that a third member of the opiorphin family of genes (ProLI) can modulate erectile physiology. We previously showed that changes in the expression of another human member of this gene family (hSMR3A) and a rat homologue (Vcsa1) modify normal erectile physiology [1,3]. In addition we now provide evidence that not only hSMR3 [1], but also ProL1 can act as a marker for ED in human patients.
The rat homologue of the opiorphin family of genes, Vcsa1, was previously shown to be down-regulated in several aetiological models of ED, a rat model for ageing, a neurogenic model (cavernous nerve transaction) and streptozotocin-induced diabetes [3,16]. In humans reporting ED, hSMR3 is down-regulated compared to patients without ED, and the present study shows that ProL1 is also down-regulated in patients with ED. In the rat submandibular gland, transcriptional regulation of rat Vcsa1 expression is under adrenergic control [2,4] and the release of the mature pentapeptide product, sialorphin, is regulated by adrenaline [17]. It is possible that in humans both hSMR3 and ProL1 are under the same regulatory mechanisms in corporal smooth muscle tissue. The expression of opiorphin family genes in corporal tissue might therefore be secondary to other factors that result in ED, e.g. the down-regulation of androgens that occur with ageing and diabetes [18] or decreases in the levels of neurotransmitters that occur with nerve damage or transaction.
It was reported that the mature pentapeptide gene products of ProL1 and Vcsa1 (opiorphin and sialorphin, respectively) act as NEP inhibitors [7,8]. We showed that sialorphin can relax corporal smooth muscle tissue in organ-bath studies by a mechanism involving its action as an NEP inhibitor [6]. Given the similarity in the peptides generated by the opiorphin homologues it seems likely that all the members of the opiorphin family of genes exert their effect through a mechanism which involves inhibition of NEP. We recently noted that Vcsa1 is involved in the regulation of G-protein-coupled receptor expression, and therefore the involvement of the opiorphin family of genes in ED might involve changes in the expression of this family of genes.
Overall, these studies suggest that all members of the opiorphin family of genes will be useful markers for organic ED. Identifying such a marker, which would distinguish between organic and psychogenic ED, would have several medical applications. It could act as an objective assessment of the severity of erectile disease, and the efficacy of treatments, which would then lead to more appropriate dosage regimens for the pharmacotherapy of ED. In addition, given the association between ED and cardiovascular disease, such a marker might enable the early identification of patients at risk of cardiovascular disease and the early initiation of preventative measures. This report adds to the accumulating evidence that the opiorphins are important in male sexual function, as well as suggesting that they, and the downstream pathways which they regulate, might represent novel therapeutic targets for treating ED.
ACKNOWLEDGEMENTS
We thank Rani Sellers, DVM, PhD, and the Center for Histology, AECOM for interpretation of histological sections from the corpora. This work was supported by grants awarded by the NIH/NIDDK to Kelvin P. Davies (R21DK079594 and R01DK077665) and Arnold Melman (P01DK060037).
Abbreviations
- ED
erectile dysfunction
- ICP/BP
intracorporal pressure/blood pressure
- NEP
neutral endopeptidase
- RT
reverse-transcriptase.
Footnotes
CONFLICT OF INTEREST None declared.
REFERENCES
- 1.Tong Y, Tar M, Monrose V, DiSanto M, Melman A, Davies KP. hSMR3A as a marker for patients with erectile dysfunction. J Urol. 2007;178:338–43. doi: 10.1016/j.juro.2007.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rosinski-Chupin I, Rougeon F. A new member of the glutamine-rich protein gene family is characterized by the absence of internal repeats and the androgen control of its expression in the submandibular gland of rats. J Biol Chem. 1990;265:10709–13. [PubMed] [Google Scholar]
- 3.Tong Y, Tar M, Davelman F, Christ G, Melman A, Davies KP. Variable coding sequence protein A1 as a marker for erectile dysfunction. BJU Int. 2006;98:396–401. doi: 10.1111/j.1464-410X.2006.06247.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rosinski-Chupin I, Huaulme JF, Rougeot C, Rougeon F. The transcriptional response to androgens of the rat VCSA1 gene is amplified by both binary and graded mechanisms. Endocrinology. 2001;142:4550–9. doi: 10.1210/endo.142.10.8428. [DOI] [PubMed] [Google Scholar]
- 5.Messaoudi M, Desor D, Nejdi A, Rougeot C. The endogenous androgenregulated sialorphin modulates male rat sexual behavior. Horm Behav. 2004;46:684–91. doi: 10.1016/j.yhbeh.2004.06.012. [DOI] [PubMed] [Google Scholar]
- 6.Davies KP, Tar M, Rougeot C, Melman A. Sialorphin (the mature peptide product of Vcsa1) relaxes corporal smooth muscle tissue and increases erectile function in the ageing rat. BJU Int. 2007;99:431–5. doi: 10.1111/j.1464-410X.2006.06577.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rougeot C, Messaoudi M, Hermitte V, et al. Sialorphin, a natural inhibitor of rat membrane-bound neutral endopeptidase that displays analgesic activity. Proc Natl Acad Sci USA. 2003;100:8549–54. doi: 10.1073/pnas.1431850100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wisner A, Dufour E, Messaoudi M, et al. Human Opiorphin, a natural antinociceptive modulator of opioiddependent pathways. Proc Natl Acad Sci USA. 2006;103:17979–84. doi: 10.1073/pnas.0605865103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Turner AJ, Isaac RE, Coates D. The neprilysin (NEP) family of zinc metalloendopeptidases: genomics and function. Bioessays. 2001;23:261–9. doi: 10.1002/1521-1878(200103)23:3<261::AID-BIES1036>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- 10.Krassoi I, Pataricza J, Papp JG. Thiorphan enhances bradykinin-induced vascular relaxation in hypoxic/hyperkalaemic porcine coronary artery. J Pharm Pharmacol. 2003;55:339–45. doi: 10.1211/002235702667. [DOI] [PubMed] [Google Scholar]
- 11.Marton Z, Pataricza J, Krassoi I, Varro A, Papp JG. NEP inhibitors enhance C-type natriuretic peptide-induced relaxation in porcine isolated coronary artery. Vascul Pharmacol. 2005;43:207–12. doi: 10.1016/j.vph.2005.07.007. [DOI] [PubMed] [Google Scholar]
- 12.Andersson KE. Erectile physiological and pathophysiological pathways involved in erectile dysfunction. J Urol. 2003;170:S6–13. doi: 10.1097/01.ju.0000075362.08363.a4. [DOI] [PubMed] [Google Scholar]
- 13.Andersson KE. Pharmacology of penile erection. Pharmacol Rev. 2001;53:417–50. [PubMed] [Google Scholar]
- 14.Davies KP, Zhao W, Tar M, et al. Diabetes-induced changes in the alternative splicing of the slo gene in corporal tissue. Eur Urol. 2007;52:1229–37. doi: 10.1016/j.eururo.2006.11.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Christ GJ, Day N, Santizo C, et al. Intracorporal injection of hSlo cDNA restores erectile capacity in STZ-diabetic F-344 rats in vivo. Am J Physiol Heart Circ Physiol. 2004;287:H1544–53. doi: 10.1152/ajpheart.00792.2003. [DOI] [PubMed] [Google Scholar]
- 16.User HM, Zelner DJ, McKenna KE, McVary KT. Microarray analysis and description of SMR1 gene in rat penis in a post-radical prostatectomy model of erectile dysfunction. J Urol. 2003;170:298–301. doi: 10.1097/01.ju.0000060882.75475.5a. [DOI] [PubMed] [Google Scholar]
- 17.Rougeot C, Rosinski-Chupin I, Njamkepo E, Rougeon F. Selective processing of submandibular rat 1 protein at dibasic cleavage sites. Salivary and bloodstream secretion products. Eur J Biochem. 1994;219:765–73. doi: 10.1111/j.1432-1033.1994.tb18556.x. [DOI] [PubMed] [Google Scholar]
- 18.Seftel AD. Male hypogonadism. Part I Epidemiology of hypogonadism. Int J Impot Res. 2006;18:115–20. doi: 10.1038/sj.ijir.3901397. [DOI] [PubMed] [Google Scholar]