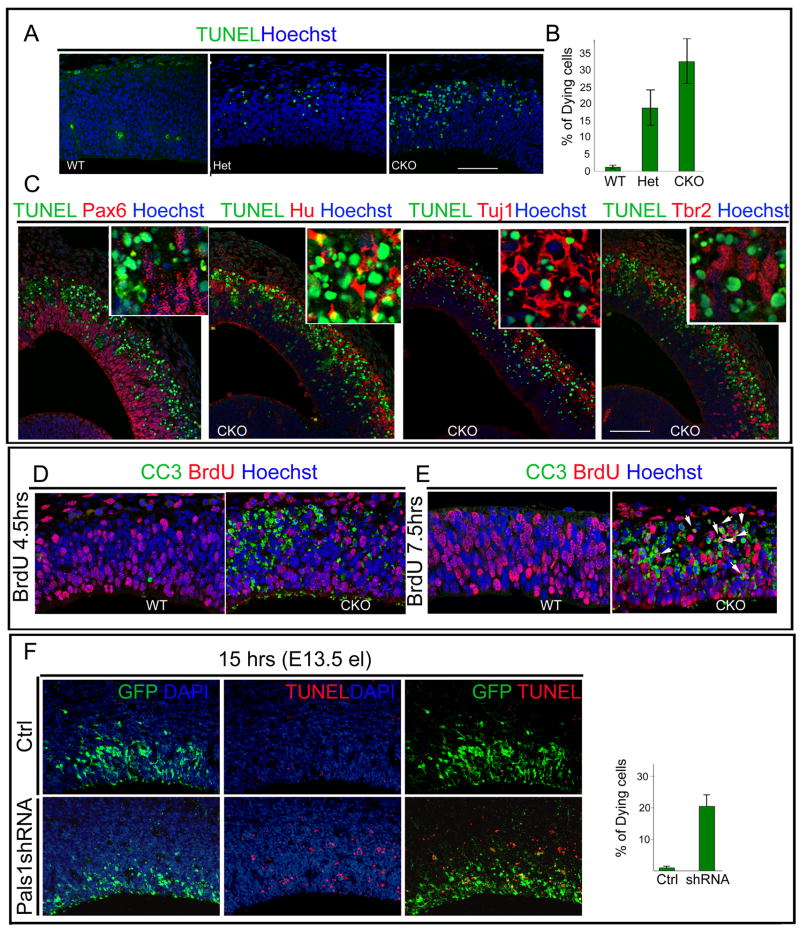
Figure 5. Pals1 deficiency causes massive and rapid cell death of abnormally generated postmitotic neurons.
(A, B) More cells in the Pals1 CKO undergo apoptotic cell death than in Pals1 Het or WT littermates, as seen by TUNEL staining at E12. Dying cells are observed by E10 and peak at E11-E12 (ANOVA; WT vs. Het p<0.05; WT vs. CKO p<0.0005; Het vs. CKO p<0.05; n=3 and n=5). (C) The cells undergoing apoptotic cell death are primarily neuronal, as shown by double-labeling with progenitor markers, (apical progenitors: Pax6; basal progenitors: Tbr2) and the nuclear neuronal markers Hu, Tuj1 together with TUNEL staining. Hu and Tuj1 extensively overlap with TUNEL-stained dying cells while progenitor markers rarely overlap with TUNEL-positive staining cells. The size markers represent 75μm. (D) The cells in M phase or G2 phase, which are labeled by a 4.5 hour BrdU pulse, rarely overlap with cleaved caspase 3 (CC3)-positive staining cells. (E) Cells labeled by a 7.5 hour BrdU pulse are mainly in G1 or exiting the cell cycle. In the Pals1 CKO brain, most BrdU-positive cells double label with CC3 (E, arrow). (F) Pals1 knockdown cells acutely undergo apoptotic cell death since over 20% of Pals1 shRNA-treated cells are TUNEL-positive as early as 15 hrs after electroporation. See also Figure S5.