Figure 4.
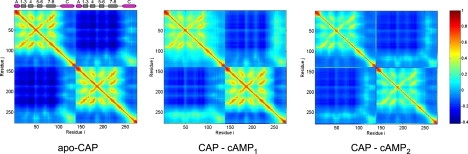
Cross-correlation map, Λij, between residue i and j, for three ligation states of CAPN, obtained from the Gaussian network model implemented on the webserver iGNM. A pair subjected to a fully correlated motion (Λij = 1) is colored dark red, fully anticorrelated motions (Λij) are not present, and moderately correlated motions are colored dark blue. cAMP binding disturbs correlations in the liganded monomer (top-left corner of the middle picture) but introduces correlation between the central helices and the liganded monomer. Binding of the second cAMP reestablishes symmetry in the motion pattern and removes correlations of the central helices to the β-sheet structures. Main parts of the secondary structure of CAP are shown above the APO-CAP map; α-helices are represented as magenta cylinders, and β-sheets as gray rectangles.
