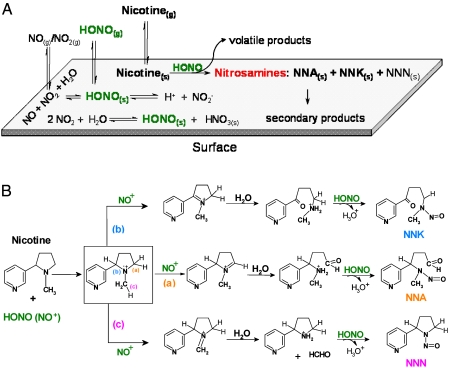
Fig. 2.
Physical-chemical processes involved in the formation of TSNAs. (A) Illustration of surface-mediated nitrosation of nicotine. HONO(s) can be formed through three pathways: (i) direct adsorption of HONO(g), (ii) heterogeneous disproportionation of NO2, and (iii) surface-catalyzed reaction between NO and NO2. HONO(s) reacts with nicotine generating NNA and NNK. NNN was also produced with lower yields. Secondary products are listed in Table 1. (B) Proposed mechanism for the formation of TSNAs. The first step involves the electrophilic attack of NO+ on nicotine, leading to the formation of the unstable cationic intermediate shown in the box. The second step is initiated with abstraction of a hydrogen atom to form an iminium cation, which is then hydrolyzed by sorbed water molecules. Finally, HONO nitrosates the secondary amines to form NNA, NNK, and NNN.