Fig. 1.
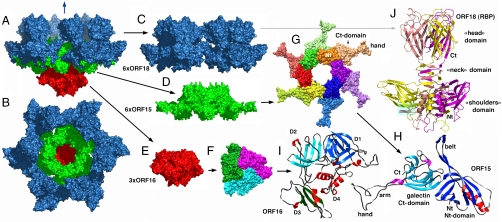
Crystal structure of the phage p2 baseplate and its components. (A) View of the baseplate surface; ORF15 is in green, ORF16 in red, and ORF18 in blue. The blue arrow indicates the position of the quasi 6-fold axis and points toward the rest of the phage tail and the capsid. (B) The baseplate has been rotated by 90° around the horizontal axis. The central channel formed by ORF15 hexamer is closed by the ORF16 trimeric dome. (C) The arrangement of ORF18 as six trimers. (D) Hexameric ORF15. (E) Trimeric ORF16. (F) View of ORF16 trimer rotated 180° relative to baseplate (B) with a different color for each subunit. (G) ORF15 hexamer is viewed in the same orientation as in (B). Each subsubunit, as well as the N- and C-terminus domains, have a different color. The central channel is ∼40 Å wide. (H) Ribbon view of ORF15 subunit. The N- and C-terminus domains have their β-strands colored dark and light blue, respectively; helices are colored red and violet, respectively; and coiled sections are gray. The striking features of the structure, the arm (which helps hexamerization) and the extension (which grips ORF18 trimer) have been identified. Note the closure of the surface at the center of the trimer. (I) Ribbon view of ORF16 subunit. The four domains have been identified by D 1 to 4 and different colors of the β-strands. Domain 4 is only helical. These domains correspond to those identified in gp27 from phage T4 (12). (J) Ribbon view of the receptor-binding protein ORF18 trimer, with a different color for each chain. The trimer domains of shoulders, neck, and head are represented. The VHHs are displayed in Fig. S2.