Fig. 1.
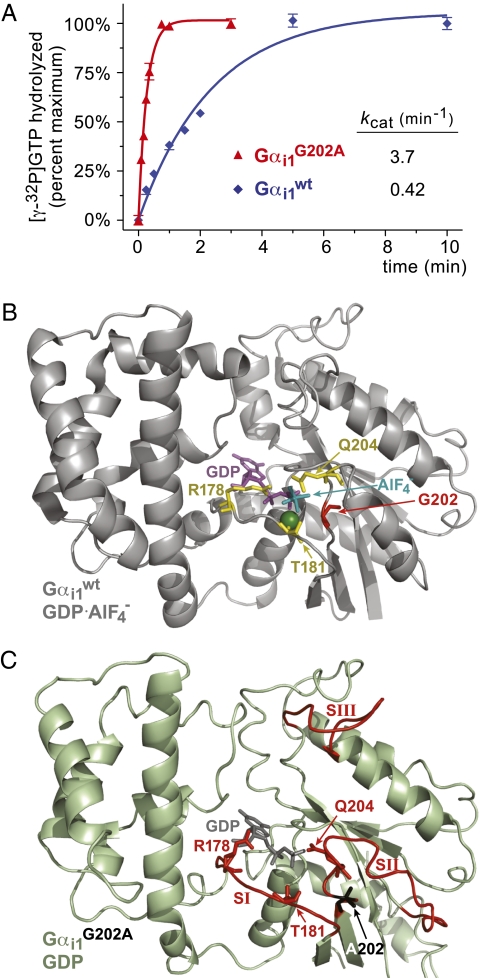
G202A substitution in Gαi1 switch II leads to a pretransition state that accelerates intrinsic GTPase activity. (A) Single-turnover GTP hydrolysis assays were performed on ice using indicated recombinant wild-type and mutant Gα proteins, demonstrating the enhanced intrinsic GTPase rate of the Gαi1(G202A) mutant. The nearly order-of-magnitude GTPase rate enhancement observed with the G202A mutation is consistent with that reported by Thomas et al. (22); nevertheless, it must be acknowledged that some RGS proteins have been observed to accelerate Gα-mediated GTP hydrolysis by orders of magnitude under optimal conditions (24, 45). (B) Ribbon Cα tracing of the proposed transition state for GTP hydrolysis from the published structural model of wild-type Gαi1·GDP·AlF4 − (PDB id 1GFI; (23), highlighting the disposition of the three residues involved in GTP hydrolysis (Arg-178, Thr-181, Gln-204; yellow sticks), as well as the position of glycine-202 (red). GDP is colored magenta with the AlF4 − and magnesium ions colored teal and green, respectively. (C) Ribbon Cα tracing of our 2.6 Å structural model of GDP-bound Gαi1(G202A) derived from x-ray crystallography (PDB id 2PZ2). Switch regions (SI-SIII) are colored red, with the catalytic residues Arg-178, Thr-181, and Gln-204 depicted in red sticks and the mutant alanine-202 residue in black sticks.