Abstract
Sonar broadcasts are followed by echoes at different delays from objects at different distances. When broadcasts are emitted rapidly in cluttered surroundings, echo streams from successive broadcasts overlap and cause ambiguity in matching echoes to corresponding broadcasts. To identify reactions to ambiguity in clutter, echolocating bats that emit multiple-harmonic FM sounds were trained to fly into a dense, extended array of obstacles (multiple rows of vertically hanging chains) while the sonar sounds the bat emitted were recorded with a miniature radio microphone carried by the bat. Flight paths were reconstructed from thermal-infrared video recordings. Successive rows of chains extended more than 6 m in depth, so each broadcast was followed by a series of echoes from multiple rows of chains that lasted up to 40 ms. Bats emitted sounds in pairs (“strobe groups”) at short (20–40 ms) interpulse intervals (IPIs) alternating with longer IPIs (>50 ms). For many short IPIs, the stream of echoes from the first broadcast was still arriving when the second broadcast was emitted. This overlap caused ambiguity about matching echoes with broadcasts. Bats shifted frequencies of the first sound in each strobe group upward and the second sound downward by 3–6 kHz. When overlap and ambiguity ceased, frequency shifts ceased also. Frequency differences were small compared with the total broadcast band, which was 75–80 kHz wide, but the harmonic structure of echoes enhances the differences in spectrograms. Bats could use time–frequency comparisons of echoes with broadcasts to assign echoes to the corresponding broadcasts and thus avoid ambiguity.
Keywords: bat sonar, clutter interference, echo delay, FM sounds
Echolocating big brown bats (Eptesicus fuscus) use sonar to guide flight and find prey in the dark (1, 2). Their sonar broadcasts are FM, sweeping downward from ≈110 kHz to 20 kHz in several harmonics (e.g., FM1, FM2, FM3) (Fig. 1A Inset) (3, 4). These bats change the interpulse intervals (IPIs), the initial high frequencies and terminating low frequencies of FM sweeps, the duration, and the amplitude of broadcasts according to surrounding conditions such as the distance to nearby objects (3–7). Each sonar broadcast impinges on objects at different distances to form a stream of echoes returning at different delays. Bats determine the distance to objects from the delay of echoes that arrive during the interval that follows each broadcast (8, 9), and they can judge the depth of the target “scene” (10) from the echo-stream duration (ESD). The bat's auditory system registers echo delays out to 30 ms or more, equivalent to distances of at least 5 m (11). The scene's composition in depth is represented faithfully by the time that elapses between each broadcast and the echoes that follow it, but only if all of the echoes for one broadcast are allowed to return before the next broadcast is transmitted. If the bat emits a new broadcast before the current stream of echoes has run its course, later echoes of the first broadcast will fall inside the interval after the second broadcast and will be assigned an erroneously short delay relative to the second broadcast, causing one or more “phantom” targets to appear at close range. The intrusion of these echoes into the second broadcast's interval creates ambiguity about which broadcast really is responsible for their occurrence. When ambiguous echoes are assigned correctly to the first broadcast, their delays are perceived as relatively long, in accordance with the real scene, but when ambiguous echoes are assigned incorrectly to the second broadcast, their delays are perceived as anomalously short. The bat might treat the resulting phantom objects as obstacles that suddenly appear in its path. When maneuvering near vegetation, the bat must react swiftly to track a flying insect or to avoid a collision with the nearest branches and therefore must emit broadcasts in rapid succession (short IPIs). In contrast, to keep itself informed about the depth of the scene (the ESD) for path planning, the bat must emit broadcasts slowly enough (long IPIs) for echoes to arrive from the farthest reaches of the scene. Echoes from background objects, or clutter, can interfere with processing of echoes from targets of interest because they all are echoes of the same broadcasts and necessarily have similar waveforms. Clutter interference is exacerbated by broadcast–echo ambiguity, creating a worst-case situation—self-generated clutter resulting from the use of too-short IPIs. The obvious effectiveness of echolocation in clutter (5, 6, 12, 13) makes it desirable to learn how bats achieve this performance. When flying in dense clutter, big brown bats emit nearly all their sonar sounds in pairs, called “strobe groups,” with shorter IPIs between members of the pairs set off by longer IPIs between one strobe group and the next (3, 8, 12, 13). The intervals between strobe groups usually are long enough to allow reception of all echoes (IPIs longer than ESDs), in effect setting a depth-of-field for echolocation (14), but the bat will experience broadcast–echo ambiguity if the shorter IPIs within strobe groups lead to overlap of echo streams from the more closely spaced broadcasts (IPIs shorter than ESDs). The key to evoking streams of strobe groups is to have clutter that is both dense, with numerous, closely spaced obstacles filling the space the bat must enter, and extended, with the array of obstacles stretching to the far end of the space. [Petrites et al. (12) used up to 151 hanging chains.]
Fig. 1.
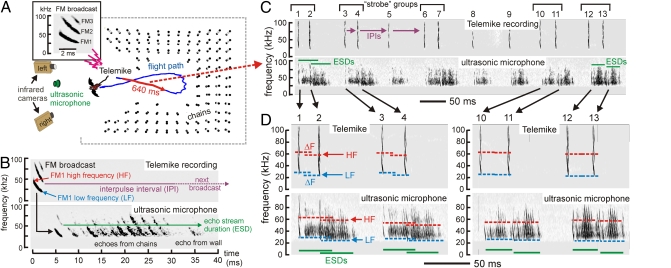
(A) Perspective plan view of chain array from above, with video-reconstructed bat flight path in blue. The red section of the blue track traces the 640-ms segment related to sounds in C and D. (Inset } Spectrogram of a typical FM big brown bat broadcast during flight (harmonics are FM1, FM2, and FM3). (B) Spectrogram of Telemike channel (Upper) shows single FM broadcast. Corresponding ultrasonic microphone channel (Lower) shows stream of echoes from chains and wall. Labels show parameters extracted from sounds (IPI, high frequency, low frequency, ESD). (C) Spectrograms of broadcasts and echoes recorded during 640-ms red-marked segment of flight at Telemike (Upper) and ultrasonic microphone (Lower). IPIs show strobe groups. The ultrasonic microphone registered smears of echoes from the chains following each broadcast. (D) Expanded view of broadcasts 1 and 2, 3 and 4, 10 and 11, and 12 and 13 to show IPIs, ESDs, and ΔFs for strobe groups. HF, high frequency; LF, low frequency.
Assessing the bat's reactions to broadcast–echo ambiguity and, more generally, to clutter, requires apparatus and methods to monitor changes in broadcasts without artifacts caused by the bat's flight movements with respect to the recording microphone. Here, we combine two recently introduced experimental methods—radio telemetry of sounds from the flying bat for very stable recording of the transmitted signals (15–17) in tandem with flight tests using an extended array of obstacles during which bats emit sounds at sufficiently short IPIs to create the conditions for ambiguity (12). The bat's response to ambiguity caused by overlap of extended echo streams is specific: Whenever ambiguity occurs (IPIs shorter than ESDs), the bat shifts the frequencies of the FM sweep upward in the first broadcast and downward in the second broadcast by several kilohertz (ΔF) in proportion to the degree of overlap (IPI minus ESD). When the bat has moved farther along its flight path, to the point at which ambiguity no longer occurs (IPIs longer than ESDs), the bat stops making this frequency shift.
Results
Occurrence of Broadcast–Echo Ambiguity During Flights in Clutter.
Both during training, when the arrangement of chains incorporated a narrow alleyway for the bat to fly entirely through the array, and during experimental measurements, when the alleyway was partly filled in to make the bat turn back, the bats successfully negotiated the obstacles and avoided collisions. On each experimental flight, the bat carrying the Telemike flew into the open area surrounded by the chain array, circled, and then flew back out. Fig. 1A shows a plan view (from above) of a sample flight path reconstructed from stereo video images during a typical trial. Video and acoustic data were taken from a total of 30 flight sessions with three bats (12, 11, and 7 flights by different bats, respectively). The spectrogram (Fig. 1A, Inset) shows a typical multiple-harmonic FM bat sound recorded by the Telemike, with harmonics labeled FM1, FM2, and FM3. The fixed ultrasonic microphone also recorded echoes in sequence from all rows of the chain array. Fig. 1B shows the spectrogram for a broadcast signal at the Telemike plus spectrograms for the same sound and its echoes acquired by the fixed ultrasonic microphone. Relevant acoustic features such as IPI, the high and low frequencies for estimating ΔF, and ESD are labeled in Fig. 2B. The data reported here are from flights into the array, before the bat turned back toward the cameras, so that the Telemike and the ultrasonic microphone could be used together to characterize the bat's behavior. Time intervals between successive chain echoes in Fig. 1B were about 2.3 ms, corresponding to the 40-cm spacing of successive rows of chains. Depending on the bat's position along its flight path (see track in Fig. 1A), chain echoes arrived over a span of time (ESD) ranging from ∼10 to ∼50 ms (mean 31.4 ms, SD 12.9) following each broadcast, culminating with an echo from the far wall (Fig. 1B). The chain array effectively replicated situations involving dense, range-extended clutter, such as a bat would encounter when flying in close proximity to vegetation (18, 19).
Fig. 2.
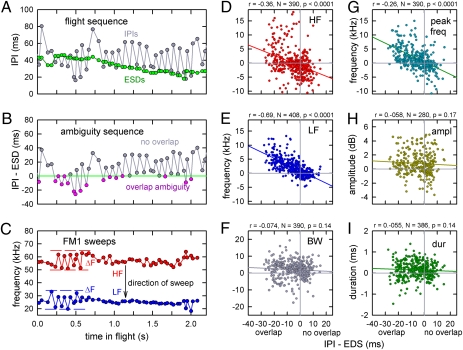
(A) Plot of successive IPIs (gray) and ESDs (green) for one representative flight. (B) Plot showing overlap (pink data-points) or nonoverlap (gray data-points) of ESDs, with consequent pulse–echo ambiguity from one sound to the next for strobe groups (negative vertical-axis values represent overlap.) (C) Plot showing high (HF) and low (LF) frequencies in FM1 for successive sounds from the same flight. When ESDs overlap, frequencies of the first sound are higher and frequencies of the second sound are lower (ΔF). Between the first and the second sound of each strobe group, plots show dependence of FM1 high frequency (HF) (D), FM1 low frequency (LF) (E), FM1 bandwidth (BW) (F), FM1 frequency at peak amplitude (G), relative sound amplitude (H), and sound duration (I) on the amount of ESD overlap. Plots in D–I are labeled with value of correlation coefficient (r), number of sound pairs (N), and significance of correlation (P).
In a typical flight (Fig. 1A), the bat emitted sonar broadcasts in strobe groups with alternating short and long IPIs (Fig. 1C; see also the sawtooth curve with gray data-points in Fig. 2A). Consequently, the distribution of IPIs across flights was bimodal, with a short IPI peak at 20–40 ms and a long IPI peak at 50–80 ms. As the bat flew farther into the array (red segment of blue track in Fig. 1A), ESDs shortened progressively (green horizontal bars in Fig. 1D) because fewer rows of chains remained in front of the bat. Across each flight, ESDs ranged from about 45 ms down to 20 ms (green data-points in Fig. 2A). IPIs between strobe groups were sufficiently long for each ESD to be completed before the next broadcast was emitted. In addition, some IPIs within strobe groups were slightly longer than ESDs, so broadcast–echo ambiguity still was avoided (gray data-points in Fig. 2B). However, especially early in the flight when the depth of the array facing the bat was greatest, IPIs within strobe groups often were shorter than ESDs (e.g., sound pairs 1 and 2 and 3 and 4 in Fig. 1D). In these instances, pulse–echo ambiguity occurred (pink data-points in Fig. 2B). Later in the flight, within-strobe-group IPIs of about the same length became longer than ESDs because the depth of the array was less (e.g., sound pairs 10 and 11 and 12 and 13 in Fig. 1D). For all broadcasts in which both IPIs and ESDs could be measured (n = 1,726), 87% had IPIs longer than ESDs, and 13% had IPIs shorter than ESDs. Most instances in which IPIs were shorter than ESDs occurred within strobe groups emitted early in the flights, and they signified the presence of pulse–echo ambiguity.
Frequency Shifts Within Strobe Groups.
During the measured segments of all 30 flights going into the chain array (i.e., left-to-right part of track in Fig. 1A), for n = 1,133 sounds, the mean high frequency of FM1 was 55.5 kHz (SD 5.0), and the mean low frequency was 25.4 kHz (SD 2.8). The mean bandwidth of FM1 by itself was 30.1 kHz (SD 5.5). Mean broadcast duration was 1.8 ms (SD 0.5). The critical comparisons for characterizing responses to pulse–echo ambiguity are between the first and second sounds in strobe-group pairs relative to the degree of overlap between ESDs in those strobe groups. Across all flights, 390 strobe-group pairs (780 broadcasts) were measurable for high frequency and 408 strobe-group pairs (816 broadcasts) were measurable for low frequency. Spectrograms in Fig. 1D reveal that the bat changes the frequencies of FM1 in each strobe-group pair of sounds by several kilohertz (ΔF) if their ESDs overlap (horizontal green bars for sounds 1 and 2 and for 3 and 4) but not if their ESDs are separated (sounds 10 and 11 and 12 and 13). (Presumably the occurrence of a long ESD following one or more broadcasts indicates to the bat the need for such a frequency shift on subsequent broadcasts; the data-points in Fig. 2 B and C show very prompt response when the IPI is shorter than the ESD.) The frequency of the FM1 of the first sound of the pair is raised, whereas the frequency of the second sound is lowered (Fig. 2C; high frequency, red data-points and curve; low frequency, blue data-points and curve), so that both the starting and the ending frequencies of FM1 harmonics in the two sounds diverge in frequency. Moreover, changes in frequency of FM1 are proportional to the amount of ESD overlap (r = −0.36 for high frequency; r = −0.69 for low frequency; for both, P < 0.0001). As would be expected, the frequency of peak amplitude in FM1 changed along with high and low frequency (Fig. 2G), but the overall bandwidth of FM1 remained unchanged (Fig. 2F). Across bats and flights, FM1 diverged by 3–6 kHz between the first and the second sounds (y-values for regression lines in Fig. 2 D, E, and G where they intersect x-values at −25 ms overlap, which is the left-hand limit of the data distribution). FM1 slid higher or lower in frequency as a unit, carrying the higher harmonics (FM2, FM3) with it, thus altering the shape of the spectrogram beyond merely introducing ΔF to FM1 alone (Fig. 3). In contrast, neither broadcast amplitude (Fig. 2H) nor broadcast duration (Fig. 2I) changed. The overall upper frequency of the broadcasts also remained fixed at about 100–105 kHz in either FM2 or FM3, corresponding to the upper-frequency limit of the bat's hearing (20), thus capping the total sonar band, including harmonics, to frequencies ranging from 100 kHz down to each sound's low frequency (about 25 kHz).
Fig. 3.
Spectrograms for (A) broadcast #3 followed by idealized echo of #3, (B) broadcast #4 followed by idealized echo of #4, and (C) broadcast #4 followed by idealized echo of #3 (from Fig. 1 C and D). Echo delay is indicated by the horizontal black arrow. Vertical blue bars on frequency axis in A–C show spectrum for echo; short red bar in C shows one-dimensional spectral mismatch (ΔF) between broadcast #4 and echo #3. Red areas on spectrogram in C show two-dimensional time–frequency mismatch between the spectrogram of echo #3 and the spectrogram of broadcast #4. Spectral mismatch is about 6% (red bar vs. blue bar + red bar); because of the harmonic structure, the spectrogram mismatch is >50% (red areas vs. black areas + red areas).
Discussion
Our observations reveal that, when ESDs overlap and pulse–echo ambiguity occurs, the measured frequencies in successive biosonar sounds are shifted away from each other by about 3–6 kHz (Fig. 2 D, E, and G), without accompanying changes in signal amplitude or duration (Fig. 2 H and I). We conclude, first, that these frequency shifts result from the bat's response, because the miniature microphone in the Telemike system protrudes forward from the Telemike circuit board on the bat's back and neck to occupy a recording position slightly forward of the external ears and just above the bat's open mouth (15–17). This location serves as a constant acoustic “vantage point” for measuring changes in FM1, so that the observed frequency differences can be attributed reasonably to the bat's vocalizations themselves. Moreover, because both broadcast amplitude and duration remain unchanged (Fig. 2 H and I), the measured frequency shifts must indeed be frequency shifts, not artifacts of interactions that might affect threshold cutoff points used to define values for low or high frequency from spectrograms.
The ΔFs that we observed (3–6 kHz) are similar in size to frequency shifts that frequency-modulating echolocating bats make in other situations where interference is likely to occur: Conspecific bats flying in proximity shift their broadcast FM1 low frequencies away from each other, presumably to prevent interference with each other's sonar (21–23). In target-detection experiments, big brown bats avoid the frequency of continuous jamming tones in the range of 18–32 kHz by changing the low frequencies of FM1 in their broadcasts (24). In these previous studies, observed ΔFs of FM1 low frequency range from about 1–5 kHz (whether FM1 high frequencies change is not reported). Our second conclusion is that the observed frequency differences of several kilohertz in FM1 constitute the big brown bat's adaptive response to possible acoustic interference. To be effective, these frequency shifts must be sufficient in size for bats to segregate one broadcast and its associated echoes from other broadcasts and their echoes and from interfering constant-frequency sounds.
The upper limit of the big brown bat's effective echolocation band is determined by the upper frequency limit of recorded broadcasts at 100–110 kHz (3, 4) (100 kHz in the Telemike recordings) in conjunction with the upper limit of the bat's hearing, which is at 100 kHz (20). The lower limit of the echolocation band is determined by the low frequencies for FM1, which are around 25 kHz unless they undergo a shift (see Fig. 2 C and E). The bat's echolocation band thus spans 75–80 kHz, so that ΔFs of 3–6 kHz are only 4–8% of total bandwidth. How do such small differences allow the bat to segregate echoes arriving during overlapping ESDs so they can be associated with their correct broadcasts? After all, more than 90% of the frequencies contained in the two FM broadcasts within each strobe group are the same.
Fig. 3 shows spectrograms for representative FM broadcasts and idealized echoes from a typical strobe group (broadcasts #3 and #4 in Fig. 1D). These plots display the sounds’ frequency sweeps on the time–frequency plane to illustrate properties that may explain how small frequency differences between broadcasts might contribute to disambiguation of overlapping ESDs. The frequencies of FM1 (typically 25–55 kHz unless shifted by ΔF) are emitted with the widest beam (25, 26), and they are the frequencies least affected by atmospheric absorption (1, 2, 27), so they arrive more-or-less intact from the chains at the bat's ears. If IPIs are longer than ESDs, no echoes will intrude into the period about 30–35 ms after any given broadcast, which has spectrograms that differ markedly from the broadcast with respect to FM1 (Fig. 3 A and B). In contrast, for strobe groups with IPIs shorter than ESDs, the bat's upward frequency shift in the first broadcast (broadcast #3) relative to the downward frequency shift in the second broadcast (broadcast #4) will always result in echoes of the first sound having slightly higher FM1 frequencies than echoes of the second sound (Fig. 3C). Recordings such as those illustrated in Fig. 1 C and D confirm that this generalization holds true for actual echoes from the chains. When echoes toward the end of the ESD for the first sound (e.g., #3) intrude into the early portion of the ESD for the second sound (e.g., #4), the incorrectly matched, ambiguous echoes (e.g., echo #3 following broadcast #4 in Fig. 3C) will be shifted upwards in frequency compared with the most recent broadcast and compared with the correctly matched echoes of the most recent broadcast (Fig. 3B). The 75–80 kHz spectrum of each broadcast (vertical blue bar on each frequency axis in Fig. 3) is changed only by the amount ΔF at the low-frequency end of FM1 (red segment of vertical blue bar in Fig. 3C) because the spectrum otherwise is continuous from each individual low frequency all the way up to 100 kHz. The raw spectral difference between correct and ambiguous echoes is quite small (7% in Fig. 3C). However, for ambiguous strobe-group pairs, the entire FM1 sweep (low frequency, high frequency, and peak frequency together) slides upward or downward by several kilohertz (Fig. 2 D, E, and G). This change affects the two-dimensional spectrograms more dramatically than the one-dimensional spectrum because the higher harmonics are linked to FM1 by integer multiples (2× for FM2, 3× for FM3). On the time–frequency plane of Fig. 3C, the harmonic sweeps in echoes of the upward-shifted first broadcast become separated from the harmonic sweeps in echoes of the downward-shifted second broadcast to create large areas in which the spectrograms of the echoes do not match (red areas in right-hand plot of Fig. 3C are more than 50% of total areas). The key is use of spectrograms to register on the time–frequency surface the consequences of small frequency shifts such as those measured in FM1 (Fig. 2 D, E, and G). These shifts are passed to the higher harmonics by the integer relations of their frequencies, magnifying the effective difference between matched echoes (#3 with #3, #4 with #4 in Fig. 3 A and B) and mismatched echoes at FM2 and FM3 (#4 with #3 in Fig. 3C).
Multiple studies have shown that various sites in the auditory pathway (e.g., inferior colliculus, auditory cortex) of frequency-modulating bats (28–31), including big brown bats (32–36), register the brief FM sweeps in biosonar broadcasts and echoes on a frequency-by-frequency basis using responses of neurons tuned to individual frequencies distributed across the 15–100 kHz band. When big brown bats are stimulated by multiple-harmonic FM sounds of 1- to 3-ms duration, comparable to those emitted and received during flights through the chains, each neuron produces approximately one spike that marks the time-of-occurrence of that cell's best excitatory frequency along the sweep (28–36). For each broadcast or each echo, individual sweeps of FM1 or FM2 evoke a series of on-responses occurring across a population of neurons having different best excitatory frequencies. For any one sound, these spikes are distributed across time (onset-spike latency) in neurons distributed across (best) frequency, to form the time–frequency plane for a kind of neuronal spectrogram (11, 30). The use of FM signals and the pattern of neuronal responses have thus prompted speculation that bats not only might use a frequency-by-frequency representation but also might exploit the advantages of true time–frequency spectrograms (1, 2, 11, 29, 30). Further investigation of how echolocating bats resist clutter interference and segregate echoes in conditions of pulse–echo ambiguity will focus on the perceptual consequences of removal or displacement of segments of FM sweeps in echoes, such as those illustrated by the red areas in Fig. 3C. Recently, big brown bats were trained to discriminate differences in the delay of electronically generated echoes between “normal” echoes containing FM1 and FM2 at the same delay (positive stimuli at fixed delay of 3.2 ms) and modified echoes with FM2 delivered 300 μs later than FM1 (“split-harmonic” echoes as negative stimuli with FM1 at 3.2 ms and FM2 at 3.5 ms at nominal zero delay difference, with additional delay of up to 1 ms to negative stimuli for descending method-of-limits psychophysics; ref. 37). The bat's delay acuity is disrupted radically: Thresholds for detecting delay differences change from ∼50 μs (as in normal echo delay-discrimination tests) to ∼800 μs in delay-discrimination tests with split-harmonic echoes (37). This result suggests that bats may experience blur in the perception of delay for mismatched echoes in ambiguous conditions to prevent the worst case of clutter interference.
Materials and Methods
Subjects.
Three big brown bats (Eptesicus fuscus, males >4 years old) were wild-caught in Rhode Island with a permit from the state's Department of Environmental Management. They were housed in individual cages at 22–23 °C and 40–50% relative humidity with free access to food (mealworms; Tenebrio larvae) and vitamin-enriched water. The day–night cycle of the room was reversed at 12-h dark/12-h light so experiments could be conducted during the daytime. Body weight was 17–20 g. Experiments complied with Principles of Animal Care, publication #86–23 (1985) of the National Institutes of Health. Procedures were approved by the Brown University Animal Care and Use Committee.
General Experimental Procedures.
The flight chamber measured 8.5 m long × 3.3 m wide × 2.4 m high. It was wrapped with copper mesh (40 threads/cm), to shield the room from external electromagnetic interference. Walls and ceiling were covered with chemically inert acoustically absorbing Sonex panels (Ilbrook Corp.), which damped background echoes. The obstacle array was made of black plastic chains (Fig. 1A) suspended vertically from the ceiling (12). Each chain was 2.4 m long (from ceiling to floor), with oval links 7 cm long and 4 cm wide, made from plastic rings 8 mm in diameter. The horizontal width of the chain array (12 columns of chains) was 3.2 m, and the horizontal depth of the array facing the bat (13 rows of chains) was 4.8 m. With its rectangular plan, the array provided the bat a maximum diagonal near-to-far distance of slightly more than 6 m (Fig. 1A). Adjacent chains within horizontal rows were spaced 25 cm apart (a bat's wingspan is 30–33 cm); the rows were spaced in depth at 40-cm intervals to deter bats from flying laterally between adjacent rows. For initial training, chains were removed from successive rows in the center of the array to create a flight alley 75 cm wide. Bats received a mealworm after they landed on the far wall (12). After training, the chain array was rearranged to fill in the farther part of the alley so that during the experiments the bat flew into the enclosed space surrounded on three sides by hanging chains (Fig. 1A). The bat was released from the near end of the room in darkness, between the two video cameras, and was allowed to fly into this space before turning back (blue track in Fig. 1A).
Flights were recorded by two synchronized thermal-imaging infrared video cameras (Indigo/“Merlin” midrange type with 25-mm lenses; FLIR Systems) that could form distinct, easily tracked video images of the bat from its own body heat as it flew along the room in complete darkness. The cameras were placed 1.4 m above the floor, separated by 2 m, and located outside the copper-mesh shielding so their internal electronics did not interfere electromagnetically with the telemetry recording. Video images were recorded at 30 frames per second on the video channel of an SIR-1000W digital instrumentation data recorder (Sony Precision Instruments). Three-dimensional coordinates of the bat's flight path were reconstructed from the stereo video recordings using Motus v2.2 motion-analysis software (Peak Performance Technologies, Inc.). The blue curve in Fig. 1A shows one representative flight path in the chain array. The shorter (640-ms) segment of this path highlighted in red refers to the spectrograms in Fig. 1 C and D.
Sound Recordings.
The bat's sonar pulses were recorded by a custom-made telemetry microphone (Telemike) mounted on the bat's upper back and head (15–17). It registered the bat's FM broadcasts (Fig. 1A) without Doppler shifts, amplitude changes, or spectral distortions using a small (3-mm diameter) omnidirectional Knowles FG-3329 electret microphone with response to above 100 kHz (i.e., beyond the upper limit of a bat's hearing; ref. 20). After fur was removed from the bat's upper back and head by brief application of Nair depilatory cream (Church and Dwight Co.), the Telemike was attached with double-sided tape, with its microphone pointing forward, between the bat's ears and ≈1 cm above the bat's mouth. This microphone's very small diameter rendered it virtually omnidirectional over the restricted range of relative motion between the bat's open mouth and the microphone itself, so that the recordings retained their fidelity even if the bat moved its head during flight. The Telemike unit weighed <0.6 g, including the battery (light enough to be carried by bats weighing as little as 6–8 g) (16). Big brown bats are robust (14–22 g) and exhibited no fatigue from carrying the Telemike on multiple flights. The Telemike transmitted bat-sound–modulated radio signals with a carrier frequency between 90 and 105 MHz to an FM radio antenna (AM/FM amplified antenna; Radio Shack Corp.) attached to the ceiling of the flight chamber. The received signals were demodulated using a custom-made FM receiver, amplified and high-pass filtered at 10 kHz, then digitized at a sampling rate of 384 kHz (16-bit accuracy) and recorded on the Sony data recorder. A second, stationary ANABAT II ultrasonic microphone (Titley Electronics, Ltd.) was placed 1.25 m above the floor and aimed toward the chain array behind the bat's flight path (Fig. 1A). The signals from this microphone also were digitized at a sampling rate of 384 kHz and recorded on the Sony data recorder, so that the sounds recorded by both microphones were synchronized with the video recording. The stationary ultrasonic microphone was more sensitive than the Telemike; it picked up echoes reflected back by the chains as well as the bat's broadcasts (Fig. 1 B–D) (15). Strong echoes were reflected by all the rows of chains over a span of 20–30 ms, corresponding to the depth of the chain array from the bat's position (Fig. 1B).
Data Analysis.
Each flight trial was digitized into a Dell Pentium-3 computer using software supplied with the data recorder (Sony Real-Time and Streamer programs). The segment containing the flight, with the sounds from the Telemike and the ultrasonic microphone, was clipped out and saved as a video file (.avi). Flight tracks of bats were reconstructed from the stereo video images using the video motion-analysis software. Acoustic characteristics of the bat's sounds were analyzed by extracting the two corresponding sound channels (Telemike and ultrasonic microphone) and displaying spectrograms using Adobe Audition v1.5 or spectrogram routines written in MatLab. IPIs, durations, amplitudes, high and low frequencies, and the frequency of the peak amplitude in FM1 were determined from the spectrograms of individual Telemike sounds (Fig. 1 C and D). Of these measures, the dimensions of FM1 (high and low frequency, frequency of peak amplitude, and duration are the most useful parameters for describing the bat's harmonically structured signals (Fig. 2 D, E, G, and I). For each sound, the frequency of peak amplitude was determined from the spectrogram of FM1, and then high and low frequencies were determined by finding the upper and lower frequencies at which FM1 amplitude had declined by 25 dB relative to this peak amplitude. The Telemike's microphone, positioned over the bat's open mouth, picked up these features effectively except for occasional sounds in which rf noise prevented measurement of all parameters. (These instances are the reason different numbers of strobe-group pairs were measured for high frequency and low frequency.) The ESD for each broadcast (Fig. 1B) was determined from the signals at the ultrasonic microphone using additional MatLab routines. During flights in dense arrays of chains, the bats most often emitted sonar pulses in pairs (“strobe groups;” Fig. 1C) that were closer together than the mean IPI (12). Then, overlap of echo streams from one broadcast to the next occurred whenever the IPI was less than the ESD.
Acknowledgments
We thank Hajime Shimamoto for his assistance, and we are grateful for the advice of two referees. This study was supported by a grant from the Ministry of Education, Culture, Sports, Science and Technology, Japan, to the Research Center for Advanced Science and Technology at Doshisha University and by Special Research Grants for the Development of Characteristic Education from the Promotion and Mutual Aid Corporation for Private Schools of Japan and the Innovative Cluster Creation Project. Further support for these experiments came from Office of Naval Research Grant N00014-04-l-0415, from National Institutes of Health Grant R01-MH069633, from the Rhode Island Space Grant (National Aeronautics and Space Administration), and S Grant S07704from the Japan Society for the Promotion of Science.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
References
- 1.Griffin DR. Listening in the Dark. New Haven, CT: Yale Univ Press; 1958. [Google Scholar]
- 2.Neuweiler G. The Biology of Bats. New York: Oxford Univ Press; 2000. [Google Scholar]
- 3.Surlykke A, Moss CF. Echolocation behavior of big brown bats, Eptesicus fuscus, in the field and the laboratory. J Acoust Soc Am. 2000;108:2419–2429. doi: 10.1121/1.1315295. [DOI] [PubMed] [Google Scholar]
- 4.Saillant PA, Simmons JA, Bouffard F, Lee DN, Dear SP. Biosonar signals impinging on the target during interception by big brown bats, Eptesicus fuscus. J Acoust Soc Am. 2007;121:3001–3010. doi: 10.1121/1.2714920. [DOI] [PubMed] [Google Scholar]
- 5.Simmons JA, Eastman KM, Horowitz SS, O'Farrell MJ, Lee DN. Versatility of biosonar in the big brown bat, Eptesicus fuscus. Acoust Res Lett Online. 2001;2:43–48. [Google Scholar]
- 6.Simmons JA. Big brown bats and June beetles: Multiple pursuit strategies in a seasonal acoustic predator-prey system. Acoust Res Lett Online. 2005;6:238–242. [Google Scholar]
- 7.Jen PHS, Kamada T. Analysis of orientation signals emitted by the CF-FM bat, Pteronotus p. parnellii and the FM bat, Eptesicus fuscus during avoidance of moving and stationary obstacles. J Comp Physiol [A] 1982;148:389–398. [Google Scholar]
- 8.Moss CF, Schnitzler H-U. Behavioral studies of auditory information processing. In: Popper AN, Fay RR, editors. Hearing by Bats. New York: Springer-Verlag; 1995. pp. 87–145. [Google Scholar]
- 9.Simmons JA, et al. Auditory dimensions of acoustic images in echolocation. In: Popper AN, Fay RR, editors. Hearing by Bats. New York: Springer-Verlag; 1995. pp. 146–190. [Google Scholar]
- 10.Moss CF, Surlykke A. Auditory scene analysis by echolocation in bats. J Acoust Soc Am. 2001;110:2207–2226. doi: 10.1121/1.1398051. [DOI] [PubMed] [Google Scholar]
- 11.Simmons JA, et al. Auditory computations for acoustic imaging in bat sonar. In: Hawkins HL, McMullen TA, Popper AN, Fay RR, editors. Auditory Computation. New York: Springer-Verlag; 1996. pp. 401–468. [Google Scholar]
- 12.Petrites AE, Eng OS, Mowlds DS, Simmons JA, DeLong CM. Interpulse interval modulation by echolocating big brown bats (Eptesicus fuscus) in different densities of obstacle clutter. J Comp Physiol [A] 2009;195:603–617. doi: 10.1007/s00359-009-0435-6. [DOI] [PubMed] [Google Scholar]
- 13.Surlykke A, Ghose K, Moss CF. Acoustic scanning of natural scenes by echolocation in the big brown bat, Eptesicus fuscus. J Exp Biol. 2009;212:1011–1020. doi: 10.1242/jeb.024620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schnitzler H-U, Moss CF, Denzinger A. From spatial orientation to food acquisition in echolocating bats. Trends Ecol Evol. 2003;18:386–394. [Google Scholar]
- 15.Hiryu S, Katsura K, Lin L-K, Riquimaroux H, Watanabe Y. Doppler-shift compensation in the Taiwanese leaf-nosed bat (Hipposideros terasensis) recorded with a telemetry microphone system during flight. J Acoust Soc Am. 2005;118:3927–3933. doi: 10.1121/1.2130940. [DOI] [PubMed] [Google Scholar]
- 16.Hiryu S, Hagino T, Riquimaroux H, Watanabe Y. Echo-intensity compensation in echolocating bats (Pipistrellus abramus) during flight measured by a telemetry microphone. J Acoust Soc Am. 2007;121:1749–1757. doi: 10.1121/1.2431337. [DOI] [PubMed] [Google Scholar]
- 17.Hiryu S, Shiori Y, Hosokawa T, Riquimaroux H, Watanabe Y. On-board telemetry of emitted sounds from free-flying bats: Compensation for velocity and distance stabilizes echo frequency and amplitude. J Comp Physiol [A] 2008;194:841–851. doi: 10.1007/s00359-008-0355-x. [DOI] [PubMed] [Google Scholar]
- 18.Müller R, Kuc R. Foliage echoes: A probe into the ecological acoustics of bat echolocation. J Acoust Soc Am. 2000;108:836–845. doi: 10.1121/1.429617. [DOI] [PubMed] [Google Scholar]
- 19.Yovel Y, Franz MO, Stilz P, Schnitzler H-U. Plant classification from bat like echolocation signals. PLOS Comput Biol. 2008;4:e1000032. doi: 10.1371/journal.pcbi.1000032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Koay G, Heffner HE, Heffner RS. Audiogram of the big brown bat (Eptesicus fuscus) Hear Res. 1997;105:202–210. doi: 10.1016/s0378-5955(96)00208-0. [DOI] [PubMed] [Google Scholar]
- 21.Miller LA, Degn HJ. The acoustic behavior of four species of vespertilionid bats studied in the field. J Comp Physiol [A] 1981;142:67–74. [Google Scholar]
- 22.Ulanovsky N, Fenton MB, Tsoar A, Korine C. Dynamics of jamming avoidance in echolocating bats. Proc R Soc Lond B Biol Sci. 2004;271:1467–1475. doi: 10.1098/rspb.2004.2750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gillam EH, Ulanovsky N, McCracken GF. Rapid jamming avoidance in biosonar. Proc R Soc Lond B Biol Sci. 2007;274:651–660. doi: 10.1098/rspb.2006.0047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bates ME, Stamper SA, Simmons JA. Jamming avoidance response of big brown bats in target detection. J Exp Biol. 2007;211:106–113. doi: 10.1242/jeb.009688. [DOI] [PubMed] [Google Scholar]
- 25.Hartley DJ, Suthers RA. The sound emission pattern of the echolocating bat, Eptesicus fuscus. J Acoust Soc Am. 1989;85:1348–1351. doi: 10.1121/1.395684. [DOI] [PubMed] [Google Scholar]
- 26.Ghose K, Moss CF. The sonar beam pattern of a flying bat as it tracks tethered insects. J Acoust Soc Am. 2003;114:1120–1131. doi: 10.1121/1.1589754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lawrence BD, Simmons JA. Measurements of atmospheric attenuation at ultrasonic frequencies and the significance for echolocation by bats. J Acoust Soc Am. 1982;71:585–590. doi: 10.1121/1.387529. [DOI] [PubMed] [Google Scholar]
- 28.Pollak GD, Marsh DS, Bodenhamer R, Souther A. Characteristics of phasic-on neurons in the inferior colliculus of anaesthetized bats with observations related to mechanisms for echoranging. J Neurophysiol. 1977;40:926–941. doi: 10.1152/jn.1977.40.4.926. [DOI] [PubMed] [Google Scholar]
- 29.Bodenhamer RD, Pollak GD. Time and frequency domain processing in the inferior colliculus of echolocating bats. Hear Res. 1981;5:317–355. doi: 10.1016/0378-5955(81)90055-1. [DOI] [PubMed] [Google Scholar]
- 30.Suga N, Olsen JF, Butman JA. Specialized subsystems for processing biologically important complex sounds: Cross-correlation analysis for ranging in the bat's brain. Cold Spring Harb Symp Quant Biol. 1990;55:585–597. doi: 10.1101/sqb.1990.055.01.056. [DOI] [PubMed] [Google Scholar]
- 31.Casseday JH, Covey E. A neuroethological theory of the operation of the inferior colliculus. Brain Behav Evol. 1996;47:311–336. doi: 10.1159/000113249. [DOI] [PubMed] [Google Scholar]
- 32.Ferragamo MJ, Haresign T, Simmons JA. Frequency tuning, latencies, and responses to FM sweeps in the inferior colliculus of the echolocating bat, Eptesicus fuscus. J Comp Physiol [A] 1998;182:65–79. doi: 10.1007/s003590050159. [DOI] [PubMed] [Google Scholar]
- 33.Sanderson MI, Simmons JA. Neural responses to overlapping FM sounds in the inferior colliculus of echolocating bats. J Neurophysiol. 2000;8:1840–1855. doi: 10.1152/jn.2000.83.4.1840. [DOI] [PubMed] [Google Scholar]
- 34.Sanderson MI, Simmons JA. Target representation for naturalistic echolocation sequences in single unit responses from the inferior colliculus of big brown bats. J Acoust Soc Am. 2005;118:3352–3361. doi: 10.1121/1.2041227. [DOI] [PubMed] [Google Scholar]
- 35.Sanderson MI, Simmons JA. Selectivity for echo spectral interference and delay in the auditory cortex of the big brown bat Eptesicus fuscus. J Neurophysiol. 2002;87:2823–2834. doi: 10.1152/jn.00628.2001. [DOI] [PubMed] [Google Scholar]
- 36.Ma X, Suga N. Corticofugal modulation of the paradoxical latency shifts of inferior collicular neurons. J Neurophysiol. 2008;100:1127–1134. doi: 10.1152/jn.90508.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Stamper SA, Bates ME, Benedicto D, Simmons JA. Role of broadcast harmonics in echo delay perception by big brown bats. J Comp Physiol [A] 2008;195:79–89. doi: 10.1007/s00359-008-0384-5. [DOI] [PubMed] [Google Scholar]