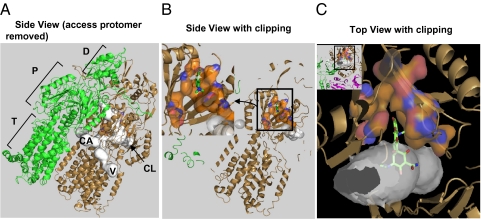
Fig. 2.
An overview of the substrate-binding pocket in the Binding protomer of AcrB. (A) The side view of the asymmetric trimer of AcrB (21), with the Access protomer removed, so that the putative substrate tunnels (detected by using the program Caver (41) and shown in white) in the Binding protomer (in the bronze color) can be seen. The three possible points of entry for substrates are shown: CL (periplasmic cleft), V (vestibule between protomers, close to the membrane surface), and CA (a large internal cavity in the transmembrane domain). The Extrusion protomer is shown in green. The porter (P) and the TolC-docking subdomains (D) of the periplasmic domain, as well as the transmembrane domain (T) are also identified. (B) The view similar to A, but the proximal portion clipped away, to reveal the binding pocket composed of F136, V139, F178, I277, A279, E280, P285, Y327, F610, V612, F615, F617, I626, and F628, shown as surface with carbons in orange. The minocycline molecule cocrystallized in PDB file 2DRD (21) is shown as green sticks. (Inset) The enlargement of the binding pocket, in a view similar to Figs. 3 and 4 and Figs. S1 and S2. (C Inset) A top view, in which the proximal part is clipped away. The Access protomer is shown in mauve. The binding pocket, shown enlarged in C, is a narrow extension of the substrate tunnel, as shown. A part of minocycline is in the pocket, whereas the hydrophilic portion protrudes into the large tunnel.