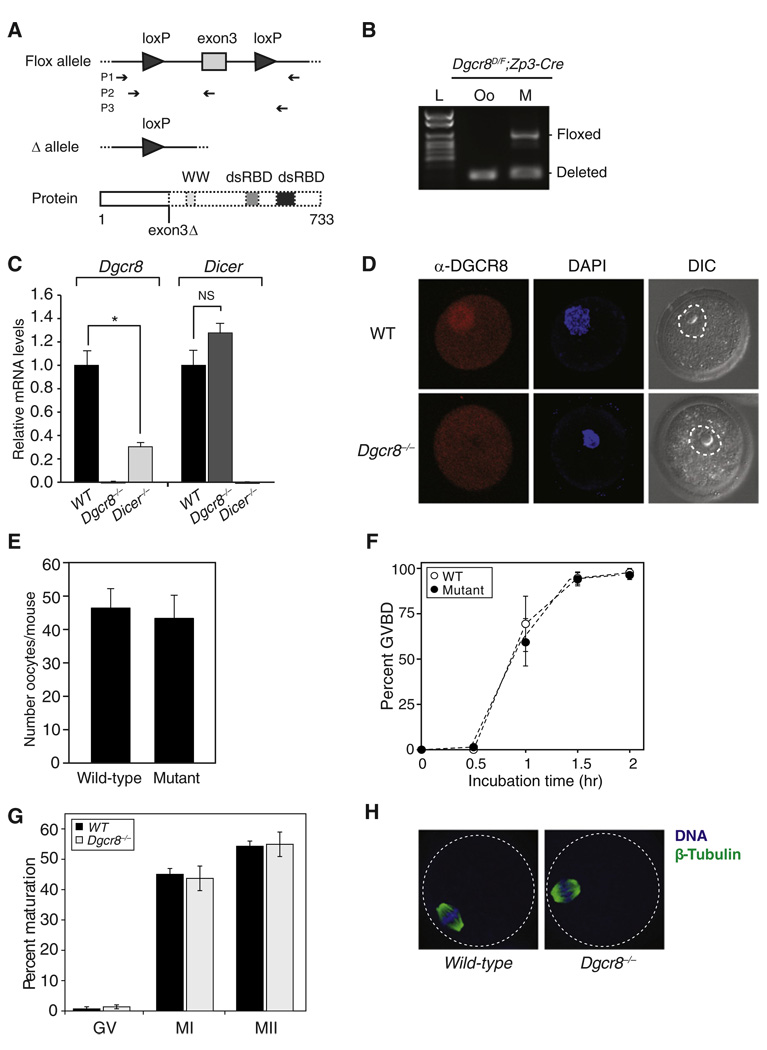
Figure 1. Dgcr8-Deficient Oocytes Mature Normally.
(A) Schematic of Dgcr8 knockout strategy. Exon 3 deletion on Dgcr8 mRNA leads to a frameshift mutation and premature stop codons, which are predicted to generate truncated DGCR8 protein. Top: box represents exon; connecting line represents intron; arrowhead represents lox sequence; arrow sets below represent primer pairs used for genotyping. Bottom: thick boxes represent the proteins that can be produced; dashed boxes represent predicted truncation; WW represents a WW protein-protein interaction domain; dsRBD represents double-stranded RNA-binding domains.
(B) Genotyping of Dgcr8delta/flox; Zp3-Cre female (M, mother) and the metaphase II (MII) oocyte (Oo) recovered from this female. Floxed alleles were detected only in mother. L denotes ladder.
(C) Dgcr8 (left) or Dicer (right) mRNA levels in wild-type, Dgcr8, and Dicer knockout oocytes. Quantitative reverse transcriptase-polymerase chain reaction (qRT-PCR) was performed. Error bars denote standard error of the mean (SEM) of three experiments. *p < 0.05; NS, not significant.
(D) Confocal microscopic images of wild-type and Dgcr8−/− oocytes stained with antibody to DGCR8. Germinal vesicle (GV) oocytes were collected, permeabilized, and incubated with DGCR8 antibody (red). DNA was visualized with DAPI (blue), and morphology was determined by differential interference contrast (DIC). Dashed line surrounds the nuclear membrane.
(E) Number of MII oocytes recovered from Dgcr8delta/flox; Zp3-Cre (mutant) or wild-type female mice, which were primed with gonadotropins. Error bar denotes SEM of seven experiments.
(F) Time course of meiotic resumption. Fully grown GV oocytes were collected from wild-type and mutant mice and scored for GV breakdown. Each point is the mean ± SEM of three experiments.
(G) The oocyte maturation status was scored by the presence of a GV (GV oocyte), a polar body (MII oocyte), or neither (MI oocyte) in wild-type and Dgcr8−/− oocytes. At least three independent pools of oocytes were scored. Error bars denote SEM of seven experiments.
(H) Spindle morphology in wild-type (left) and Dgcr8−/− (right) oocytes. Oocytes were stained with a β-tubulin antibody (green), and DNA was counterstained with DAPI (blue) (dashed line surrounds the oocyte).