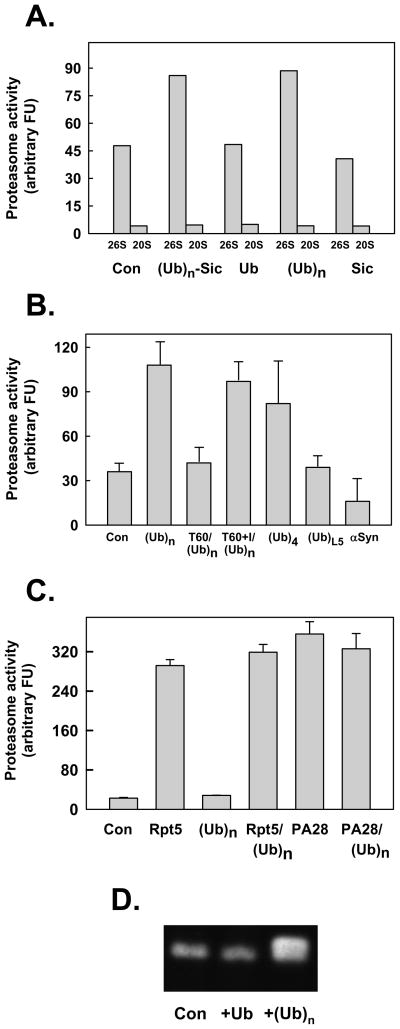
Figure 2. Polyubiquitin stimulates peptidase activity of the 26S proteasome.
Panel A. 26S proteasome (0.7 nM) or 20S proteasome (2 nM) were assayed for peptidase activity using Suc-Leu-Leu-Val-Tyr-AMC as substrate. Assays contained either no other additions (Con) or: polyubiquitylated-Sic (Ubn-Sic, 350 nM); monoubiquitin (Ub, 1 mM); K48-linked polyubiquitin (350 nM), or; Sic (50 nM). Panel B. 26S proteasome (0.7 nM) was assayed for peptidase activity using Suc-Leu-Leu-Val-Tyr-AMC as substrate. Assays contained either no other additions (Con) or: K48-linked polyubiquitin (Ubn, 340 nM); K48- linked tetraubiquitn (Ub4, 400 nM); linear tandom head-to-tail fusion of five ubiquitins (UbL5, 400 nM); and α synuclein (α– syn, 200 nM). In two other assays, 26S proteasome (0.7 nM) and K48-linked polyubiquitin (Ubn, 350 nM) were preincubated for 60 min in the absence (T60) or presence (T60+I) of ubiquitin aldehyde (10 μM) and o-phenanthroline (5 mM) prior to assay. Panel C. 20S proteasome (2 nM) was assayed for peptidase activity using Suc-Leu-Leu-Val-Tyr-AMC as substrate. Assays contained either no other additions (Con) or: Rpt5 (10 residue peptide of the C-terminus of Rpt5 PA700 subunit, 400 μM); K48-linked polyubiquitin (350 nM); 400 μM Rpt5 peptide and 350 nM K48-linked polyubiquitin; PA28 (200 nM); PA28 (200 nM) and K48-linked polyubiquitin (350 nM). For Panels A-C, data are expressed as mean values of triplicate assays (+/− SD); similar results were obtained in at least three independent experiments. Panel D. Purified 26S proteasome was preincubated briefly with ubiquitin (Ub), K48-linked polyubiquitin chains (Ubn), or buffer (Con), and then subjected to native PAGE. After electrophoresis, the gel was incubated with Suc-Leu-Leu-Val-Tyr-AMC for 15 mins at 37°C and exposed to UV light to reveal AMC product.