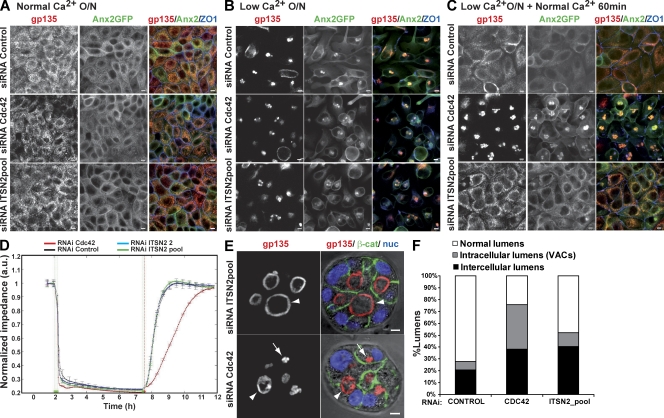
Figure 3.
Apical exocytosis is affected in Cdc42 KD cells but not in ITSN2 KD cells. (A–C) Effect of Cdc42 and ITSN2 silencing on gp135 and Anx2 exocytosis in calcium switch experiments. Anx2-GFP cells were transfected with Cdc42 siRNA (middle), ITSN2 siRNA pool (bottom) or control siRNA (top) and plated after 48 h to form a confluent monolayer (A). Cells were treated with calcium-free medium overnight (O/N; B) and then treated with complete medium for 60 min (C). Cells were fixed after each step and stained to detect gp135 and ZO-1. (D) Quantification of monolayer impedance. Control (black line)-, Cdc42 (red line)-, and ITSN2-silenced cells (blue and green lines) were plated on electrode plates and allowed to form a confluent monolayer. Cells were treated as described for A–C, and impedance was measured in real time to determine epithelial permeability. Values shown are mean ± SD from three different experiments. (E) Lumen formation phenotypes of Cdc42 and ITSN2 silencing. Cdc42 and ITSN2 KD cells were plated to form cysts for 72 h. Cells were stained to detect gp135 (left), β-catenin (β-cat), and nuclei (nuc). Arrows indicate intracellular lumens (vesicular apical compartments [VACs]), and arrowheads indicate intercellular lumens. (F) Quantification of cysts with normal lumens or with multiple intercellular and intracellular lumens in cells transfected with control siRNA, Cdc42 siRNA, or ITSN2 siRNA pool. Values shown are means from three different experiments (n ≥ 100 cysts/experiment; *, P < 0.005 for intracellular lumens of Cdc42 vs. control or ITSN2; **, P < 0.06 for intercellular lumens of ITSN2 vs. control; ***, P < 0.07 for intercellular lumens of Cdc42 vs. control). Bars, 5 µm.