Figure 2. Aβ binds to human plasma derived sLRP and human recombinant LRP-IV cluster but not to RAP using ELISA.
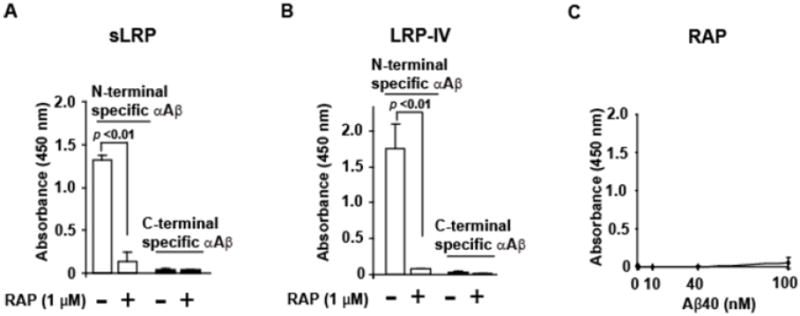
Briefly, 10 μg/ml human sLRP, recombinant LRP-IV or RAP were coated on microtiter plates and blocked with protein-free blocking buffer (Pierce 37570). For sLRP and LRP-IV, 100 nM Aβ40 was added and incubated in HBSC, pH 7.4, for 2 hours at room temperature. For RAP, various Aβ40 concentrations (0, 1, 40, 1 and 100 nM) were used. After washing with HBSC containing 0.05% Tween-20, the N-terminal specific (Cell Signaling Cat # 2454, 1μg/ml) or C-terminal specific (BA27, WAKO ELISA kit) primary antibodies were added and incubated overnight. The secondary antibody for the N-terminal anti-Aβ antibody was goat anti-rabbit (Dako; 1:2000), while the C-terminal specific primary antibody was already HRP-conjugated. The reaction was developed with 3,3′5,5′ tetramethlbenzidine (TMB; KPL,Gaithersburg, MD) and stopped with 1M HCl. Absorbance was read at 450 nm. Aβ bound to immobilized human plasma derived sLRP (A) or immobilized recombinant human LRP-IV cluster (B) in the absence or presence of RAP (1 μM) was detected using an N-terminal specific (white bars) or a C-terminal specific (black bar) anti-Aβ antibody (αAβ). C, No significant binding of monomeric Aβ40 to RAP was detected using the N-terminal specific anti-Aβ antibody. In A-C, values are mean + standard error of the mean, n=3 for each group. Pairs of groups were statistically analyzed using students t-test. These are previously unpublished data from the Zlokovic laboratory.
