Abstract
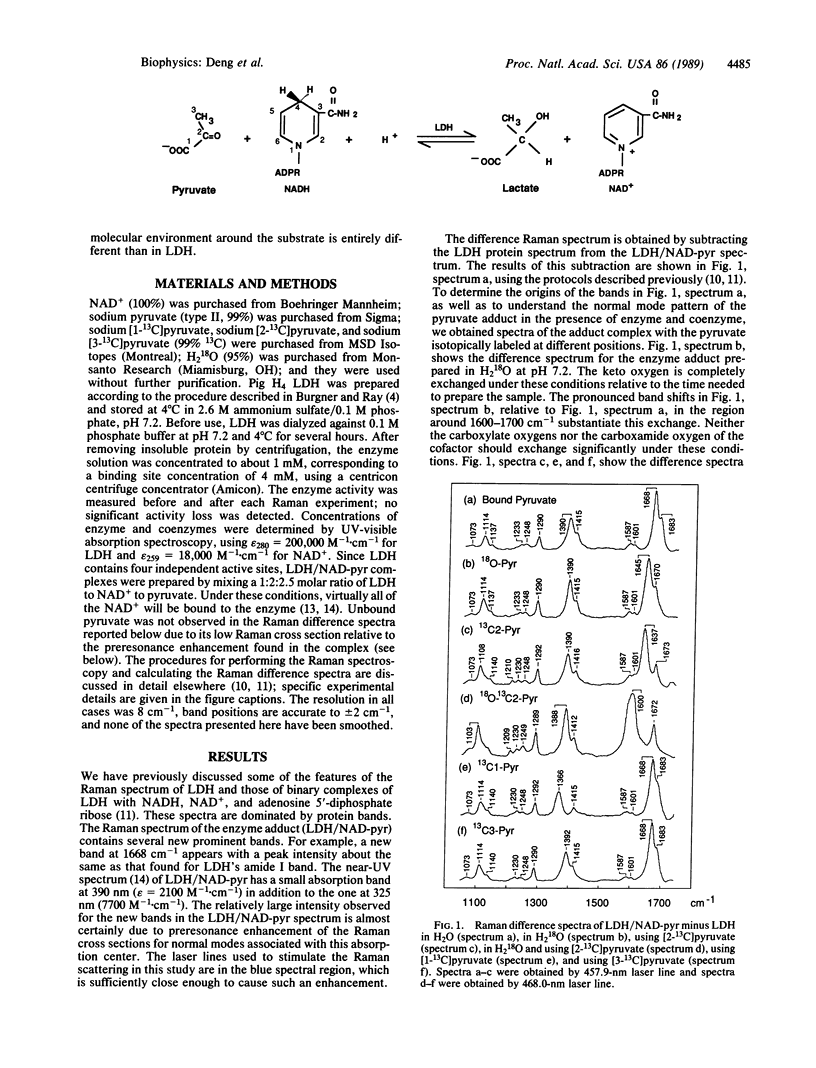
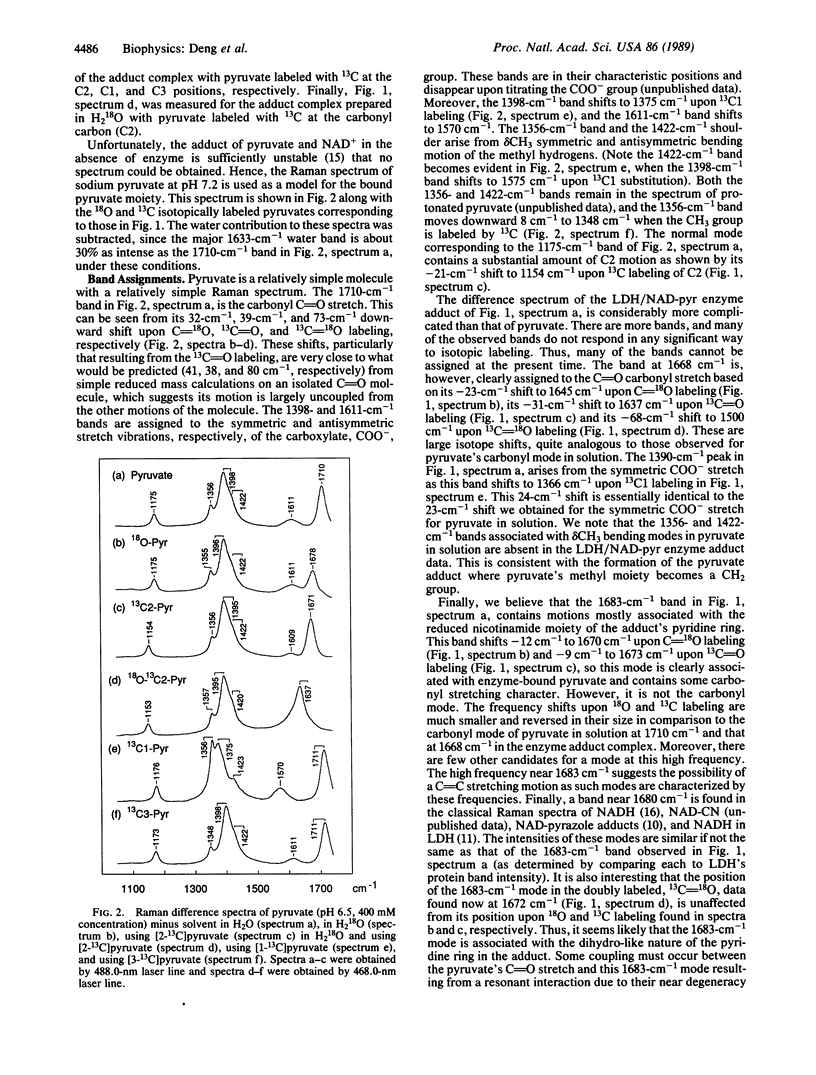
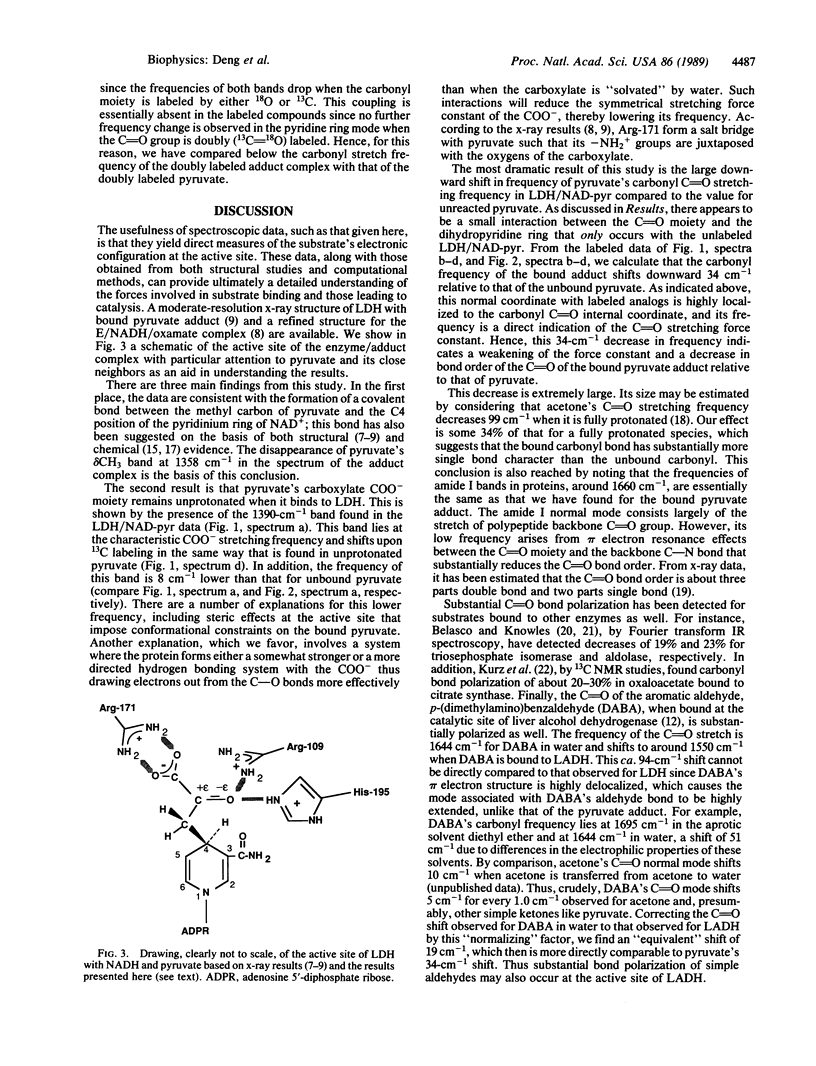
Lactate dehydrogenase (LDH; EC 1.1.1.27) catalyzes the addition of pyruvate to the four position of the nicotinamide ring of bound NAD+; this NAD-pyruvate adduct is bound tightly to the enzyme. We have used the adduct as a model for pyruvate in a competent ternary complex by comparing the Raman spectrum of the bound adduct with that for unliganded pyruvate. To understand the observed normal modes of pyruvate both as the bound adduct and in water, we have taken the Raman spectra of a series of 13C- and 18O-labeled pyruvates. We find that the carboxylate COO- moiety of pyruvate remains unprotonated at LDH's active site and forms an ion pair complex. The frequency of pyruvate's carbonyl C = O moiety shifts from 1710 cm-1 in water downward 34 cm-1 when pyruvate binds to LDH. This frequency shift corresponds to a ca. 34% polarization of the carbonyl bond, indicates a substantial interaction between the C = O group and enzyme, and is direct evidence for and is a measure of enzyme-induced electronic perturbation of the substrate needed for catalysis. This bond polarization is likely brought about by electrostatic interactions between the carbonyl moiety and the protonated imidazole group of His-195 and the guanidino group from Arg-109. We discuss how the data bear on the enzymatic chemistry of LDH.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Belasco J. G., Knowles J. R. Direct observation of substrate distortion by triosephosphate isomerase using Fourier transform infrared spectroscopy. Biochemistry. 1980 Feb 5;19(3):472–477. doi: 10.1021/bi00544a012. [DOI] [PubMed] [Google Scholar]
- Belasco J. G., Knowles J. R. Polarization of substrate carbonyl groups by yeast aldolase: investigation by Fourier transform infrared spectroscopy. Biochemistry. 1983 Jan 4;22(1):122–129. doi: 10.1021/bi00270a018. [DOI] [PubMed] [Google Scholar]
- Burgner J. W., 2nd, Ray W. J., Jr A study of pyruvate-induced inhibition in the dogfish lactate dehydrogenase system. Mechanistic comparison with the iodination of pyruvate. Biochemistry. 1974 Sep 24;13(20):4229–4237. doi: 10.1021/bi00717a025. [DOI] [PubMed] [Google Scholar]
- Burgner J. W., 2nd, Ray W. J., Jr Acceleration of the NAD cyanide adduct reaction by lactate dehydrogenase: the equilibrium binding effect as a measure of the activation of bound NAD. Biochemistry. 1984 Jul 31;23(16):3620–3626. doi: 10.1021/bi00311a008. [DOI] [PubMed] [Google Scholar]
- Burgner J. W., 2nd, Ray W. J., Jr Mechanistic study of the addition of pyruvate to NAD+ catalyzed by lactate dehydrogenase. Biochemistry. 1978 May 2;17(9):1654–1661. doi: 10.1021/bi00602a012. [DOI] [PubMed] [Google Scholar]
- Burgner J. W., 2nd, Ray W. J., Jr On the origin of the lactate dehydrogenase induced rate effect. Biochemistry. 1984 Jul 31;23(16):3636–3648. doi: 10.1021/bi00311a010. [DOI] [PubMed] [Google Scholar]
- Callender R., Chen D., Lugtenburg J., Martin C., Rhee K. W., Sloan D., Vandersteen R., Yue K. T. Molecular properties of p-(dimethylamino)benzaldehyde bound to liver alcohol dehydrogenase: a Raman spectroscopic study. Biochemistry. 1988 May 17;27(10):3672–3681. doi: 10.1021/bi00410a023. [DOI] [PubMed] [Google Scholar]
- Chen D., Yue K. T., Martin C., Rhee K. W., Sloan D., Callender R. Classical Raman spectroscopic studies of NADH and NAD+ bound to liver alcohol dehydrogenase by difference techniques. Biochemistry. 1987 Jul 28;26(15):4776–4784. doi: 10.1021/bi00389a027. [DOI] [PubMed] [Google Scholar]
- Clarke A. R., Wigley D. B., Chia W. N., Barstow D., Atkinson T., Holbrook J. J. Site-directed mutagenesis reveals role of mobile arginine residue in lactate dehydrogenase catalysis. Nature. 1986 Dec 18;324(6098):699–702. doi: 10.1038/324699a0. [DOI] [PubMed] [Google Scholar]
- Clarke A. R., Wilks H. M., Barstow D. A., Atkinson T., Chia W. N., Holbrook J. J. An investigation of the contribution made by the carboxylate group of an active site histidine-aspartate couple to binding and catalysis in lactate dehydrogenase. Biochemistry. 1988 Mar 8;27(5):1617–1622. doi: 10.1021/bi00405a034. [DOI] [PubMed] [Google Scholar]
- Deng H., Zheng J., Sloan D., Burgner J., Callender R. Classical Raman spectroscopic studies of NADH and NAD+ bound to lactate dehydrogenase by difference techniques. Biochemistry. 1989 Feb 21;28(4):1525–1533. doi: 10.1021/bi00430a016. [DOI] [PubMed] [Google Scholar]
- Everse J., Barnett R. E., Thorne C. J., Kaplan N. O. The formation of ternary complexes by diphosphopyridine nucleotide-dependent dehydrogenases. Arch Biochem Biophys. 1971 Apr;143(2):444–460. doi: 10.1016/0003-9861(71)90230-x. [DOI] [PubMed] [Google Scholar]
- Grau U. M., Trommer W. E., Rossmann M. G. Structure of the active ternary complex of pig heart lactate dehydrogenase with S-lac-NAD at 2.7 A resolution. J Mol Biol. 1981 Sep 15;151(2):289–307. doi: 10.1016/0022-2836(81)90516-7. [DOI] [PubMed] [Google Scholar]
- Kurz L. C., Ackerman J. J., Drysdale G. R. Evidence from 13C NMR for polarization of the carbonyl of oxaloacetate in the active site of citrate synthase. Biochemistry. 1985 Jan 15;24(2):452–457. doi: 10.1021/bi00323a031. [DOI] [PubMed] [Google Scholar]
- Ozols R. F., Marinetti G. V. The structure of an NAD-pyruvate complex. Biochem Biophys Res Commun. 1969 Mar 10;34(5):712–718. doi: 10.1016/0006-291x(69)90797-9. [DOI] [PubMed] [Google Scholar]
- White J. L., Hackert M. L., Buehner M., Adams M. J., Ford G. C., Lentz P. J., Jr, Smiley I. E., Steindel S. J., Rossmann M. G. A comparison of the structures of apo dogfish M4 lactate dehydrogenase and its ternary complexes. J Mol Biol. 1976 Apr 25;102(4):759–779. doi: 10.1016/0022-2836(76)90290-4. [DOI] [PubMed] [Google Scholar]
- Wolfenden R. Transition state analog inhibitors and enzyme catalysis. Annu Rev Biophys Bioeng. 1976;5:271–306. doi: 10.1146/annurev.bb.05.060176.001415. [DOI] [PubMed] [Google Scholar]
- Yue K. T., Yang J. P., Martin C. L., Lee S. K., Sloan D. L., Callender R. H. Raman study of reduced nicotinamide adenine dinucleotide bound to liver alcohol dehydrogenase. Biochemistry. 1984 Dec 18;23(26):6480–6483. doi: 10.1021/bi00321a032. [DOI] [PubMed] [Google Scholar]


