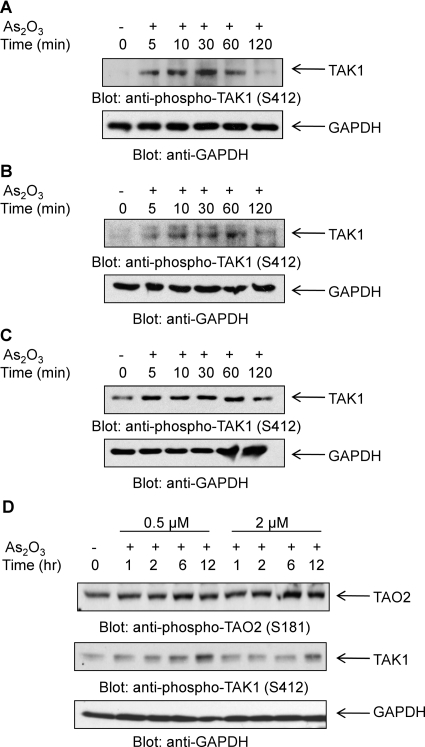
Fig. 2.
As2O3-dependent phosphorylation of TAK1 in leukemic cell lines. A, NB4 cells were incubated in the absence or presence of As2O3 (2 μM) for the indicated times. Equal amounts of total cell lysates were resolved by SDS-PAGE and immunoblotted with an anti-phospho-TAK1 (Ser412) antibody (top). The same blot was reprobed with an anti-GAPDH antibody to control for protein loading (bottom). B, as in A, but using KT-1 cells. C, as in A, but using NB4.306 cells. D, KT-1 cells were incubated in the absence or presence of As2O3 at varying times and concentrations as indicated. Equal amounts of total cell lysates were resolved by SDS-PAGE and immunoblotted with an anti-phospho-TAO2 (Ser181) antibody (top) or an anti-phospho-TAK1 (Ser412) antibody (middle). The same blot was reprobed with an anti-GAPDH antibody to control for protein loading (bottom).