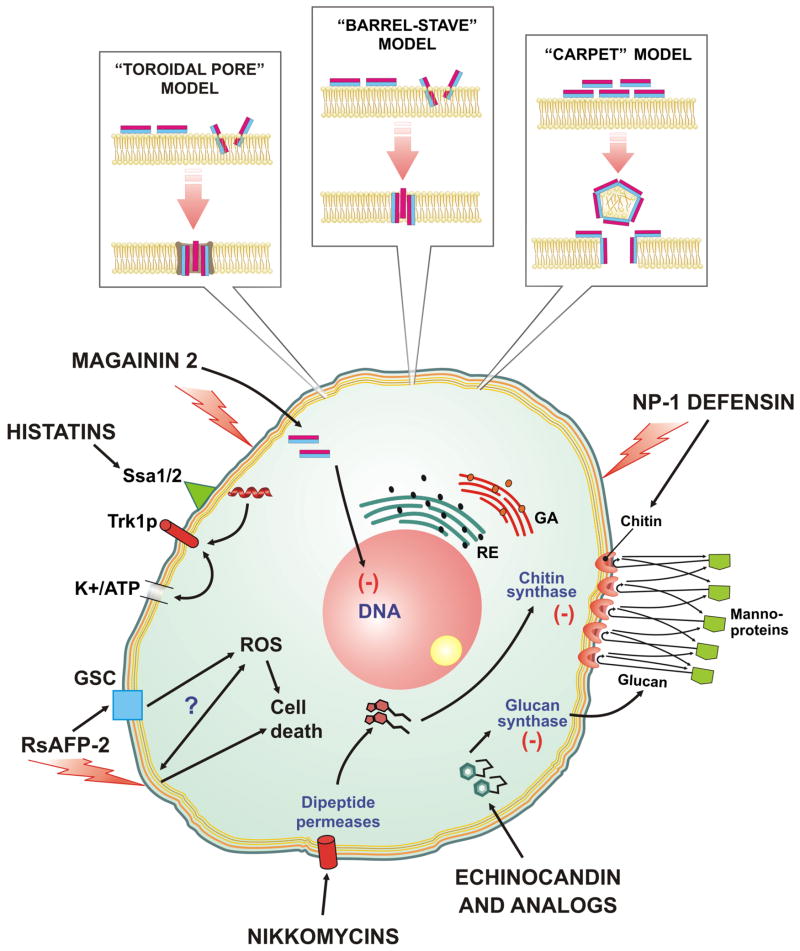
Figure 1. Modes of action proposed for anti-fungi peptides.
The majority of anti-microbial peptides inhibit filamentous fungi and yeasts by membrane lysis: three mechanisms (the toroidal pore, barrel-stave, and carpet) have been proposed to induce lysis of the membranes by AMP. In addition, there are other AMP that are more selective in their antifungal activity. Several anti-fungi peptides disrupt function and/or structure of the cell wall. Defensin NP-1 binds tightly to chitin in the cell wall and this may be important in mediating selective membrane lysis. In addition, chitin biosynthesis is blocked by nikkomycins which enter fungal cells via dipeptide permeases. The lipopeptide echinocandin and its synthetic analogs inhibit synthesis of 1–3 β glucans, a key component of the cell wall. For histatins, one mechanistic concept is based on specific binding with extracellular receptor like Ssa1/2, internalization, and binding to K+ channel transporter Trk1p and as a consequence, efflux of ATP and potassium ions from the cell. MAG-2 magainin kills fungi via cell membrane disruption and DNA damage. The plant defensin, RSAFP-2, causes membrane permeabilization via interaction with glucosylceramide (GSC) and formation of reactive oxygen species (ROS). M – mitochondria; N – nucleus; E – endosomes; L – lysosomes; RE – endoplasmic reticulum; GA – Golgi apparatus.