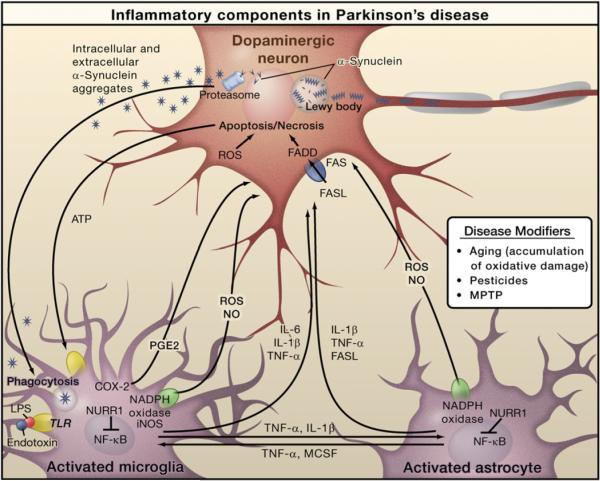
Figure 2. Inflammation in Parkinson's Disease.
Prominent neuropathological hallmarks of Parkinson's disease (PD) are the loss of dopaminergic neurons in the substantia nigra of the midbrain and the presence of intracellular inclusions containing aggregates of the α-synuclein protein, called Lewy bodies. Besides forming Lewy bodies, aggregates of α-synuclein form intermediate-state oligomers that when released from neurons activate microglia through Toll-like receptor (TLR)-independent mechanisms. This leads to activation of NF-κB and production of reactive oxygen species (ROS) and proinflammatory mediators. These factors act directly on dopaminergic neurons of the substantia nigra, which are the principal (although not the only) neurons that die in PD. These factors also activate microglia, which amplify the inflammatory response in a positive feedback loop, leading to further activation of microglia. Products derived from microglia and astrocytes act in a combinatorial manner to promote neurotoxicity. Bacterial lipopolysaccharide (LPS), acting primarily through TLR4 expressed by microglia, is sufficient to induce an inflammatory response in the substantia nigra that results in loss of dopaminergic neurons. The transcription factor NURR1 acts to suppress inflammatory responses in microglia and astrocytes by inhibiting NF-κB target genes.