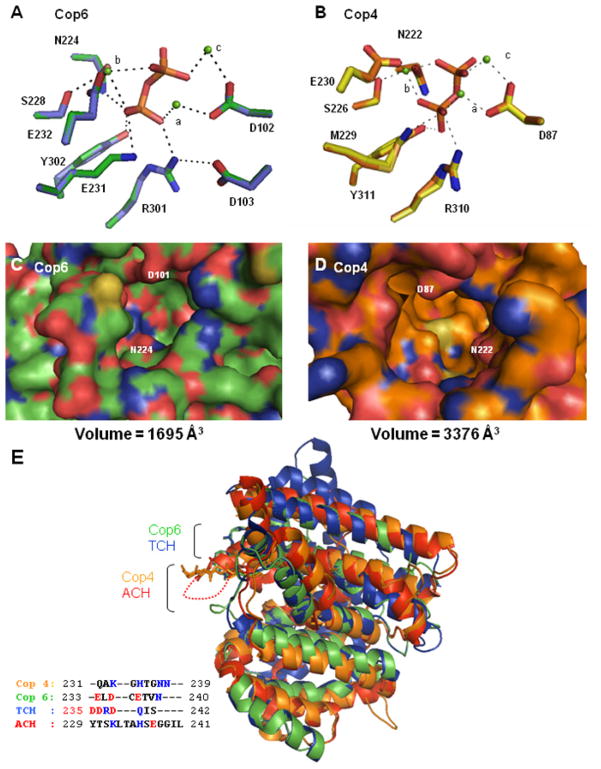
Figure 3. Structural modeling of Cop4 and Cop6 based on the structures of aristolochene synthase (ACH) [20] and trichodiene synthase (TCH) [48], respectively.
Top panels: Hydrogen bond and metal coordination interactions in the enzyme-Mg2+-PPi-complex for the Cop6 (A) (green side chains) and Cop4 (B) (orange side chains) models. Depicted side chains of the conserved DDXXD/E, NSE/DTE and basic motif (XRY) are superimposed with corresponding side chains from their respective template structures (TCH, purple; ACH, yellow). Mg2+ ions are shown in green and complexed PPi is located in the center of the networks. Center panels: View into the active site cavities of unliganded Cop6 (C) (green alpha carbons) and Cop4 (D) (orange alpha carbons). Positively charged and negatively charged amino acid residues are shown in blue and red respectively. Bottom panel: Superimposition of the unliganded Cop4 and Cop6 models with the unliganded structures of THC and ACH (E). Loops covering the active sites of the enzymes are labeled and colors correspond to those of their alpha carbon backbones in the superimposition. Inset shows the amino acid residues of the different loops. Basic (blue) and acidic (red) amino acid residues are highlighted.