Figure 5.
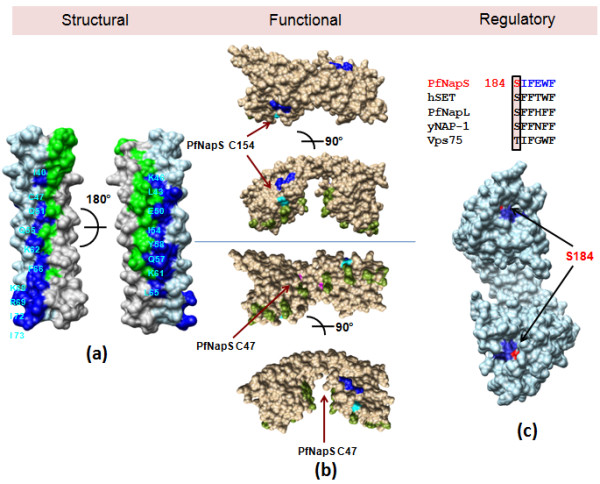
Overall structural, functional and regulatory comparisons between NAP/SET proteins based upon 5 crystal structures [7-12]. (a). PfNapS dimerization helix α2 is shown as molecular surface. Conserved dimer contributing residues between all 5 crystal structures are mapped onto PfNapS. Residues from chains A and B are coloured blue and green respectively and residues from chain A are labelled. (b) hSET is shown as molecular surface and coloured brown. Residues from hSET mutagenesis analysis are coloured green and corresponding residues from PfNapL mutagenesis are coloured blue. The corresponding residues for the two cysteines from PfNapS fluorescence data are coloured cyan and are indicated with arrows. (c) PfNapS dimer is shown as molecular surface and coloured sky blue and the conserved hydrophobic motif is coloured blue. Surface exposed serine residue is coloured red.
