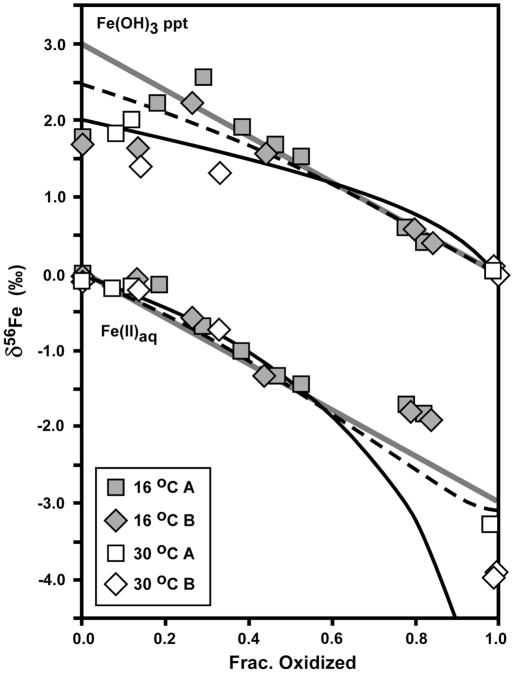
Fig. 4.
Isotopic data for Fe(II)aq and Fe(OH)3 precipitate produced by Fe(II) oxidation by Acidovorax sp. strain BoFeN1, as a function of fraction oxidized (Table 1). δ56Fe values normalized to a system value of zero. Reference curves shown for a) Rayleigh fractionation using a net Δ56FeFe(OH)3 – Fe(II)aq fractionation of +2 ‰ (black curve), b) the Rayleigh fractionation curve modified to account for partial isotopic re-equilibration (dashed black curve), and c) closed-system equilibrium fractionation for Δ56FeFe(OH)3 – Fe(II)aq = +3 ‰ (straight grey line). Partial isotopic re-equilibration calculated assuming a reactive Fe(III) surface layer of 1 nm thickness (10 nm diameter spherical particle) maintains isotopic equilibrium with Fe(II)aq. δ56Fe value for Fe(II)aq calculated in the partial re-equilibration model at increments of 1 % oxidation, using the isotope mass balance equation (eq. 4) from Crosby et al. (2007), modified to ignore sorbed Fe(II).