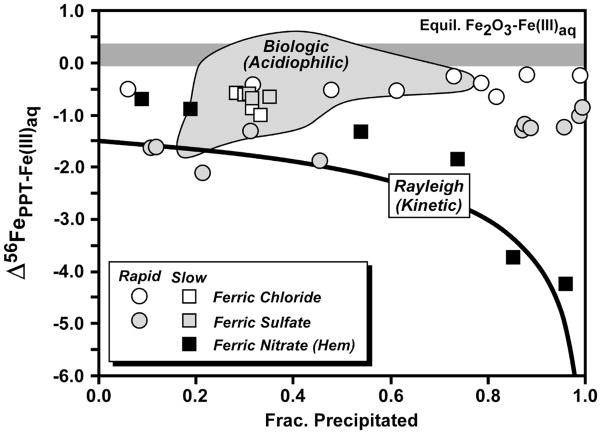
Fig. 7.
Compilation of fractionations measured between ferric oxide/hydroxide and Fe(III)aq from the studies of Skulan et al. (2002) and Balci et al. (2006). Skulan et al. estimated a slightly positive equilibrium hematite – Fe(III)aq fractionation at 98 °C (horizontal dark grey field, and determined a kinetic hematite – Fe(III)aq fractionation at 98°C (black squares) that can be described by a Rayleigh process and a Δ56FeHematite – Fe(III)aq fractionation between −1 and −1.5 ‰ (black curve shown for −1.5 ‰). The fractionation between ferric hydroxide precipitates and co-existing Fe(III)aq shown for abiologic precipitation experiments (symbols) and measured in biologic oxidation experiments (light grey field) also shown from the study of Balci et al. (2006).