Abstract
Background & Aims
Regulatory T cells (Tregs) express the forkhead box transcription factor (FoxP3) and suppress the anti-tumor immune response. We investigated whether the intratumoral densities of FoxP3+ and effector CD3+ lymphocytes are associated with prognosis of patients with colon cancer.
Methods
FoxP3 and CD3 expression and location were determined in stage II and III colon carcinomas (n=160) and normal mucosa (n=25) by immunohistochemistry; CD4 and FoxP3 were localized by dual immunofluorescence microscopy. T-cell markers were compared with patients’ pathological variables, DNA mismatch repair (MMR) status, and survival using Cox proportional hazards models.
Results
FoxP3+ and CD3+ T-cell densities increased in carcinomas compared to autologous normal mucosa (p< 0.0001). An increase in intraepithelial FoxP3+ cells was associated with poor tumor differentiation (p=0.038) and female gender (p=0.028) and advanced age of patients (p=0.042). FoxP3+ cell density was not prognostic, yet patients with tumors with reduced intraepithelial CD3+ T-cell densities had reduced disease-free survival (DFS) times (hazard ratio [HR] 1.87 [1.10, 3.16]; p=0.018). A low intraepithelial CD3+:FoxP3+ cell ratio predicted reduced DFS (46.2% vs. 66.7% survival at 5 years; HR 2.17 [1.11, 4.23]; p= 0.0205). The prognostic impact of these markers was maintained when tumors were stratified by MMR status. By multivariate analysis, a low CD3+:FoxP3+ cell ratio (p=0.0318) and low numbers of CD3+ T cells (p=0.0397) predicted shorter DFS times and were stronger prognostic variables than tumor stage or number of lymph node metastases.
Conclusions
A low intraepithelial CD3+:FoxP3+ cell ratio and reduced numbers of CD3+ T cells were associated with reduced patient survival time, indicating the importance of an effector to Treg cell ratio in colon cancer prognosis.
Introduction
Colorectal cancer (CRC) ranks fourth in incidence and second in mortality among malignancies within the United States1. Once solid tumors develop, tumor-associated antigens (TAAs) elicit a host-mediated immune response characterized by tumor infiltrating T lymphocytes (TILs). The majority of TILs in human colon cancers are CD3+ T cells that mediate the adaptive immune response. However, human cancers employ immune-evasion strategies that can cause failure of tumor-directed immune rejection. Such strategies include the actions of regulatory T cells (Treg) that contribute to cancer-related immunosuppression2–4. Treg trafficking and expansion have been shown to prevent the induction of TAA-specific immunity2. Tregs express the forkhead box nuclear transcription factor (FoxP3) that is required for their development and function5–7. FoxP3 is a marker for Tregs and its expression can be detected in tissues using immunohistochemistry. Prior studies using flow cytometry have identified and defined Tregs as CD4+CD25+FoxP3+ T cells2. CD25 forms the high affinity IL-2 receptor complex and is predominantly expressed by activated effector CD4+ T cells and by Tregs8. Expression of FoxP3 is sufficient to confer suppressive activity on naive T cells6, 9, 10. Gain-of-function, overexpression studies, and analysis of Scurfy (sf) mice deficient in FoxP3 have shown that FoxP3 is essential for the development and maintenance of Tregs6, 11. Both mice and humans lacking functional FoxP3 develop autoimmune diseases that can be prevented by transfer of normal CD4+CD25+ T cells, thus indicating the crucial role of FoxP3 in the regulation of Treg function and immune surveillance9. These experiments with adoptively transferred human12 and mouse13 Tregs provide a direct link between Tregs and reduced tumor immunity.
Tregs were found to be significantly increased in the peripheral blood of cancer patients compared to healthy controls14. Furthermore, increased Tregs have been detected in varying types of cancer supporting a role for Tregs in cancer-induced immunosuppression14–17. Tregs found in the tumor microenvironment represent thymus-derived, naturally-occurring Tregs and Tregs from conversion of CD4+CD25− T cells2. Studies indicate that depletion of intratumoral Tregs can induce potent T cell tumor immunity resulting in the regression of large established tumors in a murine model18. FoxP3+ Treg were associated with adverse outcome in human ovarian12, 19, breast20, hepatocellular21, and gastric carcinomas15. However, conflicting data exist in ovarian carcinoma22, and FoxP3+ TILs were not prognostic in renal cell carcinomas23 nor in esophageal cancers24. It is currently unknown as to whether Tregs can influence clinical outcome in human colon cancer patients. In a prior study in colon cancers, we demonstrated the prognostic impact of TILs without defining T cell sub-populations or Tregs within the peritumoral inflammatory infiltrate25. The role of TILs in cancer prognosis is controversial and may reflect the inclusion or exclusion of stromal versus intraepithelial lymphocytes among studies, as well as the lack of stratification of cases based upon DNA mismatch repair (MMR) status in CRCs26–28. While the majority of CRCs develop via an allelic loss pathway, approximately 10–15% show defective MMR29 that is associated with increased immunogenicity as evidenced by a higher density of TILs compared to tumors with intact MMR25, 26, 30. Furthermore, CRCs with defective MMR have a more favorable clinical outcome compared to tumors with intact MMR in spite of being higher grade31, 32.
To gain an increased understanding of the role of Tregs in immune evasion and prognosis, we analyzed the density and location of FoxP3+ and CD3+ TILs in human colon carcinomas. Associations were sought with clinicopathological features including MMR status, and with clinical outcome. Evaluation of Tregs and the level of immune activation in the immune microenvironment in primary tumors may be of prognostic utility, and may assist in the development or use of neoadjuvant or adjuvant treatment strategies, including immunotherapy.
Materials & Methods
Patient Specimens
Surgically resected, TNM stage II and III primary colon adenocarcinomas (n= 160) were analyzed from patients who participated in 5-fluorouracil (5-FU)-based adjuvant chemotherapy trials conducted by Mayo Clinic and the North Central Cancer Treatment Group. Available paraffin-embedded tumor blocks from a nonrandom subset of study participants were utilized. Tumor histologic grade was categorized as grade 1: well differentiated; grade 2: moderately differentiated; grade 3: poorly differentiated; grade 4: undifferentiated. Tumor site was defined relative to the splenic flexure and those at the flexure were regarded as distal. The study treatments were as follows: 5-FU and leucovorin + standard dose or high dose levamisole (91-46-53), portal venous 5-FU versus observation (79-46-04), or 5-FU + levamisole versus 5-FU + levamisole + leucovorin (89-46-51)33, 34. Of the 160 patients in our study, 144 were randomized to treatment arms and 16 received observation alone.
Immunohistochemical detection of FoxP3
Formalin-fixed tissues were deparaffinized and heat-induced epitope retrieval was performed using a steamer. After blocking peroxidase activity, slides were incubated with an anti-FoxP3 mouse monoclonal antibody [FoxP3 Abcam (ab20034)], diluted 1:50 for 30 min., in an Autostainer (DAKO, Carpenteria, CA). The secondary antibody system [Advance HRP Polymer and Advance HRP Enzyme (DAKO)] was applied for 15 min. Color was developed using the DAB chromagen followed by counterstaining in hematoxylin. For a negative control, the primary antibody was omitted but all other steps were followed. Human tonsil was used as a positive control.
CD3, CD8, and CD25 immunostaining
Antigen retrieval was performed using Target Retrieval Solution (DAKO). After blocking peroxidase activity followed by a protein block, slides were incubated with a mouse monoclonal anti-human CD3 antibody (clone F7.2.38; DAKO) at 1:100 for 30 min. After rinsing, slides were incubated in Envision + HRP kit immunoglobulins for 30 min. For CD8 staining, the mouse monoclonal anti-human CD8 antibody (clone C8/144B, DAKO) was utilized at 1:100 for 30 min, and the same procedures outlined above were utilized. For CD25, slides were incubated in Sniper Background Reducer (Biocare Medical) for 5 min., and then incubated with an anti-human CD25 antibody (R&D Systems) at 1:200 for 30 min. Incubation in Mach 3 Mouse-HRP polymer kit (Biocare) was performed for 15 min. Color was developed using DAB and then counterstaining with hematoxylin.
Immunofluorescence microscopy
Dual immunofluorescence of FoxP3 and CD4 was performed on paraffin sections using the catalyzed signal amplification (CSA) system (DAKO; Denmark). CD4 staining was performed first using a CSA kit (DAKO) and the anti-CD4 antibody (Novacastra, 1:1000 for 15 min). Sections were then incubated with biotinylated anti-mouse IgG, rinsed, and a streptavidin-biotin complex was added (15 min.) followed by incubation with biotinylated tyramide (15-min). CD4 was visualized with Texas Red-avidin. Sections were pre-treated for 2 hrs. with 0.1M L-glycine and FoxP3 staining (Abcam, Inc, 1:500 for 15 min.) was performed using the DAKO CSAII kit. Anti-mouse-IgG-HRP was added for 15 min. with rinsing in wash buffer followed by a 15-min. incubation with FITC-tyramide.
Analysis and Quantification of FoxP3 and T Cell Markers
Tissue sections containing tumor were scanned at light microscopy (10X magnification) to ascertain areas with high numbers of immunopositive FoxP3+ TILs. The number of FoxP3+ TILs per high power field (HPF) in these areas was then determined by counting 10 high-power fields (5 tumor epithelium and 5 stromal areas) and then average values were calculated. CD8 and CD25 staining were analyzed and quantified in a tumor subset (n=26) using the same criteria as for FoxP3. CD3 expression was analyzed in the same cases stained for FoxP3 that were included in tissue microarrays that had been subsequently constructed. As performed for FoxP3+, areas of high CD3+ density were selected for quantification. There were 3 tumor cores per case and the number of CD3+ lymphocytes was determined by counting 2 HPFs for each core and then data for the 3 cores was averaged. All of the specimens were analyzed and T cells counted by a pathologist (RLR) without knowledge of any clinical information. Prior to scoring, a limited number of randomly selected cases were reviewed with a second gastrointestinal pathologist to establish the scoring criteria for the immune markers.
DNA Mismatch Repair Status
The status of the DNA mismatch repair (MMR) system was determined as previously described35. Tumors were classified into three groups: 1) microsatellite stable (MSS) with no MSI at any of the loci examined, 2) low instability (MSI-L, <30% of the loci demonstrating MSI), or 3) high instability (MSI-H, > or equal 30% of the loci demonstrating MSI35. In one of the adjuvant studies, defective MMR was defined by instability at the mononucleotide BAT 26 locus coupled with absent expression of an MMR protein36, 37. For purposes of our analysis, MMR-deficient cases included those with MSI-H and MMR-proficient included MSS and MSI-L cases.
Statistical analysis
Biomarker expression was dichotomized for DFS and OS. Chi-square or Fisher’s exact tests were used to test for an association between prognostic markers for categorical variables. The Wilcoxon rank-sum test was used to test for an association between dichotomized markers and continuous variables, and to test for differences in paired continuous data collected on the same patients. OS (censored at 8 yr) was calculated as the number of years from randomization to the date of death or last contact. DFS (censored at 5 yr) was calculated as the number of years from randomization to the first of either disease recurrence or death. Distributions of OS and DFS were estimated using Kaplan-Meier methodology. Univariate and multivariate Cox proportional hazards models were used to explore the association of clinical and biomarker expression with OS and DFS. Score and likelihood ratio test p-values were used to test the significance of each covariate in the univariate and multivariate models, respectively. Graphical and statistical methods were used to examine whether underlying model assumptions were satisfied (e.g., proportional hazards). Statistical tests were two sided, with P ≤ 0.05 considered significant. Statistical analyses were performed using SAS software (SAS Institute, Cary, NC).
Results
Study Population
TNM stage II and III [136 (85%)] primary colon adenocarcinomas (n= 160) were analyzed from patients who participated in 5-fluorouracil (5-FU)-based adjuvant chemotherapy trials (see Methods). After 5 years of follow-up, 70.6% of patients were alive and at 8 years, 60.6% remained alive. Details of the study population are shown in Table 1.
Table 1.
Univariate Analysis of Clinicopathological Variables, T Lymphocytes Markers, and Disease-Free (DFS) and Overall Survival (OS).
| Parameter | Total (N) |
5-yr DFS % |
Hazard Ratio (95% CI) |
P-value1 | 5-yr OS % |
Hazard Ratio (95% CI) |
P-value |
|---|---|---|---|---|---|---|---|
| Stage | |||||||
| II | 24 | 75.0% | - | 0.2164 | 83.3% | - | 0.1485 |
| III | 136 | 61.0% | 1.70 (0.73,3.96) | 68.4% | 1.85 (0.79,4.29) | ||
| Lymph Node Number2 | |||||||
| 0 | 24 | 75.0% | - | 0.0394 | 83.3% | - | 0.0153 |
| 1–3 | 91 | 68.9% | 1.38 (0.57,3.31) | 75.7% | 1.48 (0.62,3.53) | ||
| >3 | 45 | 50.0% | 2.51 (1.01,6.20) | 59.5% | 2.81 (1.14,6.91) | ||
| Histologic Grade | |||||||
| Well/moderate | 101 | 62.4% | - | 0.8527 | 71.3% | - | 0.5409 |
| Poor/undifferentiated | 59 | 64.4% | 0.95 (0.56, 1.62) | 69.5% | 1.17 (0.71, 1.94) | ||
| Tumor Site | |||||||
| Distal | 75 | 60.0% | - | 0.3051 | 70.7% | - | 0.3130 |
| Proximal | 85 | 65.9% | 0.76 (0.45, 1.28) | 70.6% | 0.77 (0.47, 1.28) | ||
| Age | |||||||
| <70 | 106 | 66.0% | - | 0.3969 | 74.5% | - | 0.0271 |
| ≥70 | 54 | 57.3% | 1.26 (0.74,2.13) | 63.0% | 1.75 (1.06,2.88) | ||
| Gender | |||||||
| Female | 76 | 61.8% | - | 0.7772 | 69.7% | - | 0.6917 |
| Male | 84 | 64.3% | 0.93 (0.56, 1.55) | 71.4% | 0.90 (0.55, 1.49) | ||
| FoxP3+ Intraepithelial | |||||||
| ≤ Quartile 1 | 59 | 66.1% | 0.81 (0.47, 1.39) | 0.4414 | 74.6% | 0.73 (0.43, 1.24) | 0.2385 |
| > Quartile 1 | 101 | 61.4% | - | 68.3% | - | ||
| FoxP3+ Stromal | |||||||
| ≤ Quartile 1 | 42 | 61.9% | 1.15 (0.63,2.08) | 0.6555 | 66.7% | 1.10 (0.62, 1.95) | 0.7512 |
| > Quartile 1 | 118 | 63.5% | - | 72.0% | - | ||
| CD3+ Intraepithelial | |||||||
| ≤ Quartile 1 | 47 | 51.1% | 1.87 (1.10,3.16) | 0.0180 | 63.8% | 1.46 (0.87,2.46) | 0.1492 |
| > Quartile 1 | 113 | 68.1% | - | 73.5% | - | ||
| CD3+ Stromal | |||||||
| ≤ Quartile 1 | 40 | 70.0% | 0.67 (0.35, 1.26) | 0.2096 | 77.5% | 0.70 (0.38, 1.30) | 0.2612 |
| > Quartile 1 | 120 | 60.8% | - | 68.3% | - | ||
| CD3+/Foxp3+ | |||||||
| Intraepithelial1 | |||||||
| ≤ Quartile 1 | 26 | 46.2% | 2.17 (1.11,4.23) | 0.0205 | 61.5% | 1.76 (0.92,3.37) | 0.0868 |
| > Quartile 1 | 75 | 66.7% | - | 70.7% | - | ||
| CD3+/Foxp3+ Stromal2 | |||||||
| ≤ Quartile 1 | 38 | 68.4% | 0.78 (0.41, 1.47) | 0.4406 | 73.7% | 0.99 (0.55, 1.77) | 0.9664 |
| > Quartile 1 | 117 | 61.5% | - | 69.2% | - | ||
CD3+/Foxp3+ ratio for 101 patients with Foxp3+ TILs in tumor epithelium (i.e. counts > 0)
CD3+/Foxp3+ ratio for 155 patients with Foxp3+ TILs in tumor stroma (i.e. counts > 0)
FoxP3+ TILs in Human Colon Carcinomas
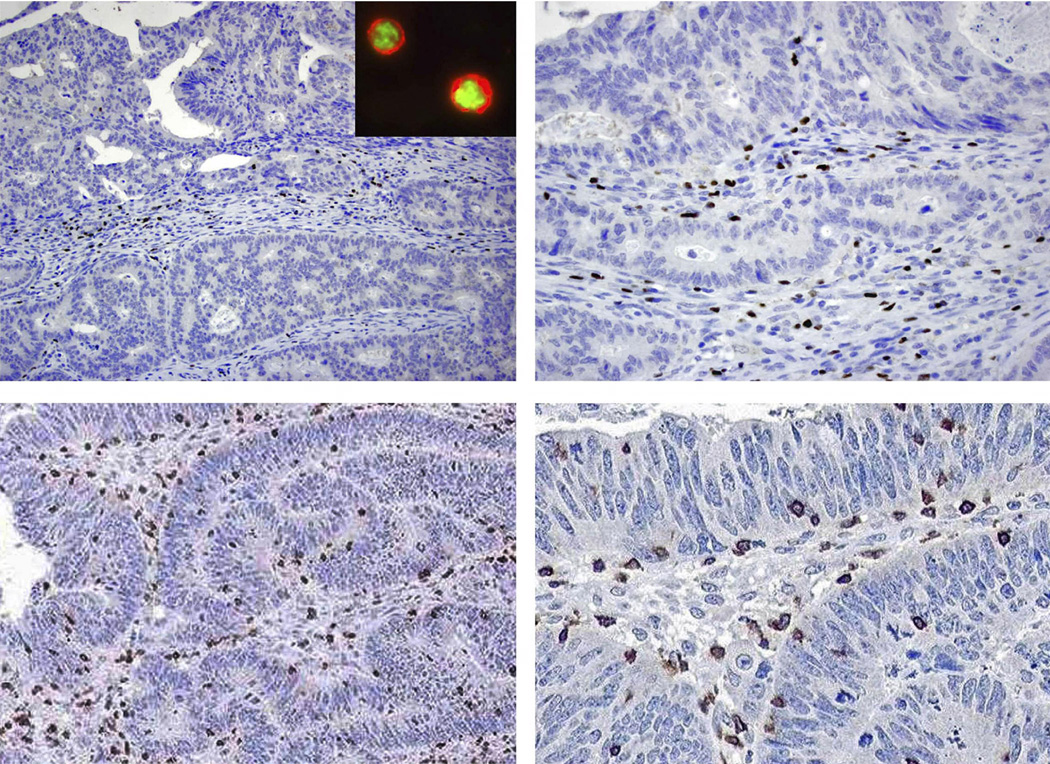
We analyzed Tregs, identified by FoxP3+ staining, and the adaptive immune marker CD3+ in colon carcinomas (Figure 1). Further evidence that our FoxP3+ TILs were Tregs was shown by performing dual staining for FoxP3 and CD4 in colon carcinomas using immunofluorescence microscopy (Figure 1, inset). It has been demonstrated that FoxP3+CD4+ cells are Tregs whereas FoxP3-negative CD4+ cells are largely T-helper cells38. Coexpression of FoxP3+ and CD4+ are shown whereby the FoxP3 antigen localizes to the nucleus and CD4+ shows a membranous staining pattern in the cytoplasm of T lymphocytes (Figure 1, inset). FoxP3+ and CD3+ lymphocytes invading tumor epithelium or infiltrating the peritumoral stroma were quantified and categorized separately (Figure 1). The density of these T cell markers in tumors was compared to histologically normal-appearing colonic mucosa from the same patients (n= 25). We found that the number of intraepithelial FoxP3+ lymphocytes was increased in tumors versus normal epithelia [p <0.0001]. Similarly, FoxP3+ T lymphocytes were increased in tumor stroma relative to normal lamina propria [p <0.0001].
Figure 1.
Top, FoxP3+ T lymphocytes (brown color) are seen in peritumoral stroma and infiltrating epithelial cells of a sporadic colon carcinoma using immunohistochemistry (400X). Inset, Dual staining for FoxP3+ (nuclear) and CD4+ (cytoplasm) are seen in colon carcinoma cells using immunofluorescence microscopy. Bottom, Epithelial and stromal CD3+ T lymphocytes are shown in a colon carcinoma at light microscopy (400X).
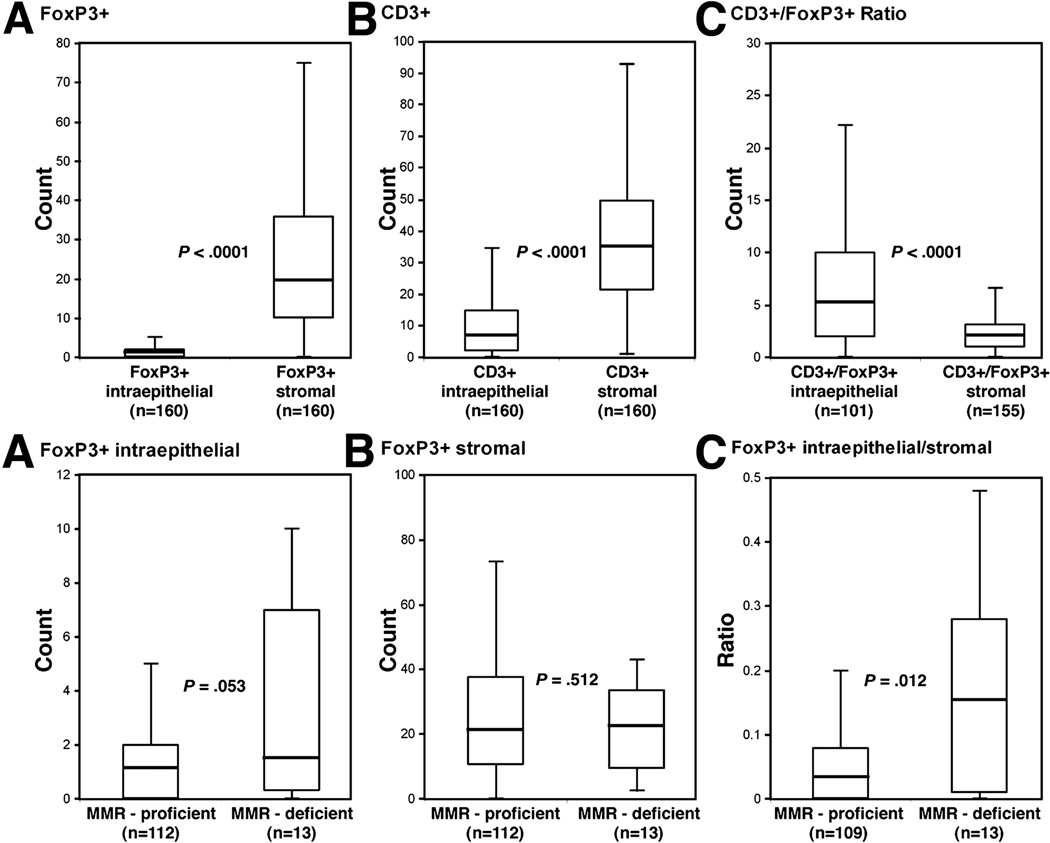
Within carcinomas, the density of FoxP3+ and CD3+ TILs was increased in the stroma compared to the epithelia (p <0.0001 for both) [Figure 2, top]. We also examined the CD3+:FoxP3+ ratio. This ratio enables a determination of the relative proportion of CD3+ cells to Tregs and excluded the possibility that changes in frequency of FoxP3+ TILs reflect nonspecific effects on total lymphocyte numbers. The CD3+:FoxP3+ ratio was higher in the tumor epithelia relative to the stroma (p-value < 0.0001, Figure 2, top).
Figure 2.
Top, density of intraepithelial versus stromal FoxP3+ (A), CD3+ (B), or the CD3+:FoxP3+ T lymphocyte ratio (C) in TNM stages II and III colon carcinomas. Bottom, comparison of the density of FoxP3+ T lymphocytes infiltrating tumor epithelia (A), stroma (B), and the epithelial to stromal ratio in DNA mismatch repair (MMR)-deficient versus –proficient colon carcinomas. Box plots show median values, interquartile ranges, and extreme values (error bars).
FoxP3+ TILs and Clinicopathological Variables
The density of intraepithelial FoxP3+ TILs was increased in poor/undifferentiated carcinomas (p=0.038), in females (p=0.028), and in elderly patients, i.e., age 70 years or older versus younger patients (p=0.042) [Table 2]. The intraepithelial CD3+ TIL density was higher in poor/undifferentiated carcinomas (p=0.030), but was unrelated to other variables (Table 2). The intraepithelial CD3+:FoxP3+ T cell ratio was unrelated to clinicopathological variables. In tumor stroma, a higher density of FoxP3+ TILs was associated with fewer metastatic LNs (p=0.044) [data not shown]. In addition, an increased stromal CD3+:FoxP3+ ratio was associated with younger patient age at colon cancer resection (p=0.03).
Table 2.
Correlation between T cell Markers and Clinicopathological Variables.
| Variable | Histologic Grade |
Gender |
Age |
||||||
|---|---|---|---|---|---|---|---|---|---|
| well/ moderate |
poor/ undifferentiated |
P value1 | Female | Male | P value1 | <70 y | ≥70 | P value1 | |
| FoxP3+ epithelium | 0.038 | 0.028 | 0.042 | ||||||
| Median (Range) | 0.6 (0 – 10) | 1.0 (0 – 11) | 1.0 (0 – 11) | 0.6 (0 – 10) | 0.7 (0 – 11) | 1.0 (0 – 10) | |||
| CD3+ epithelium | 0.030 | 0.135 | 0.373 | ||||||
| Median (Range) | 6.0 (0 – 43) | 8.0 (0 – 71) | 8.0 (0 – 71) | 6.0 (0 – 43) | 6.0 (0 – 71) | 8.0 (0 – 43) | |||
| CD3+/Foxp3+ epithelium | 0.442 | 0.306 | |||||||
| Median (Range) | 4.7 (0 – 55) | 5.9 (0 – 45) | 4.2 (0 – 55) | 5.4 (0 – 43) | 0.364 | 5.9 (0 – 50) | 4.4 (0 – 55) | ||
Wilcoxon Rank-sum test.
In our patient population, 13 of 125 (10.4%) tumors analyzed showed defective DNA mismatch repair (MMR) consistent with the frequency of this phenotype in sporadic colon cancers36, 39, 40 (Table 3). The density of intraepithelial (p=0.0530), but not stromal (p=0.512), FoxP3+ TILs was increased in MMR-deficient versus proficient tumors (Figure 2 (bottom), Table 3). Furthermore, we computed a FoxP3+ intraepithelial to stroma ratio that was found to be significantly higher in MMR-deficient cases (Figure 2, bottom). There was a trend toward a higher density of intraepithelial CD3+ TILs (p=0.0635) and an increased CD3+ epithelial to stroma ratio in MMR-deficient versus proficient tumors (Table 3). The CD3+:FoxP3+ ratio did not differ based upon MMR status [Table 3]. To gain further insight into the composition of TILs within MMR-deficient tumors, we analyzed CD8+ and CD25+ T cells. CD8+ T cells exert their cytolytic activity on tumor cells by releasing their granule components, notably granzyme B and perforin41. While no differences in CD8+ T cell density were found, MMR-deficient (vs. proficient) tumors showed a higher CD8+:FoxP3+ ratio in tumor stroma (p= 0.0513) [data not shown]. CD25 is expressed on activated, effector T cells and is also expressed by Tregs (CD4+CD25+FoxP3+). MMR-deficient tumors showed an increased density of intraepithelial CD25+ T cells (p=0.0048) whereas a higher density of stromal CD25+ T cells was found in MMR-proficient cases (p=0.0276) [Table 3]. These data indicate that MMR-deficient colon cancers have increased numbers of activated, effector T cells infiltrating the tumor epithelium.
Table 3.
T Lymphocyte Marker Localization and DNA Mismatch Repair (MMR) Status.
| Intraepithelial | Stromal | Intraepithelial/Stromal | |
|---|---|---|---|
| FoxP3+ | |||
| MMR – Deficient (n=13) | 1.4 | 21.0 | 0.15 |
| MMR – Proficient (n=112) | 1.0 | 19.8 | 0.031 |
| P-value2 | 0.053 | 0.512 | 0.012 |
| CD3+ | |||
| MMR – Deficient (n=13) | 16.0 | 39.0 | 0.44 |
| MMR – Proficient (n=112) | 6.0 | 34.5 | 0.16 |
| P-value2 | 0.064 | 0.903 | 0.069 |
| CD3+/FoxP3+ ratio | |||
| MMR – Deficient (n=10) | 5.1 | 1.93 | 3.0 |
| MMR – Proficient (n=68) | 5.0 | 1.83 | 3.8 |
| P-value2 | 0.545 | 0.740 | 0.714 |
| CD8+ | |||
| MMR – Deficient (n=13) | 29.2 | 72.4 | 0.43 |
| MMR – Proficient (n=13) | 7.6 | 77.2 | 0.11 |
| P-value2 | 0.281 | 0.538 | 0.383 |
| CD25+ | |||
| MMR – Deficient (n=12) | 10.7 | 26.0 | 0.38 |
| MMR – Proficient (n=13) | 2.0 | 44.8 | 0.05 |
| P-value2 | 0.005 | 0.028 | 0.0001 |
Median values of a calcuated index are shown (see Methods).
Wilcoxon Rank-Sum test.
3 cases excluded due to 0 values in the denominators.
13 MMR-deficient, 109 MMR-proficient.
FoxP3+ TILs and Patient Survival
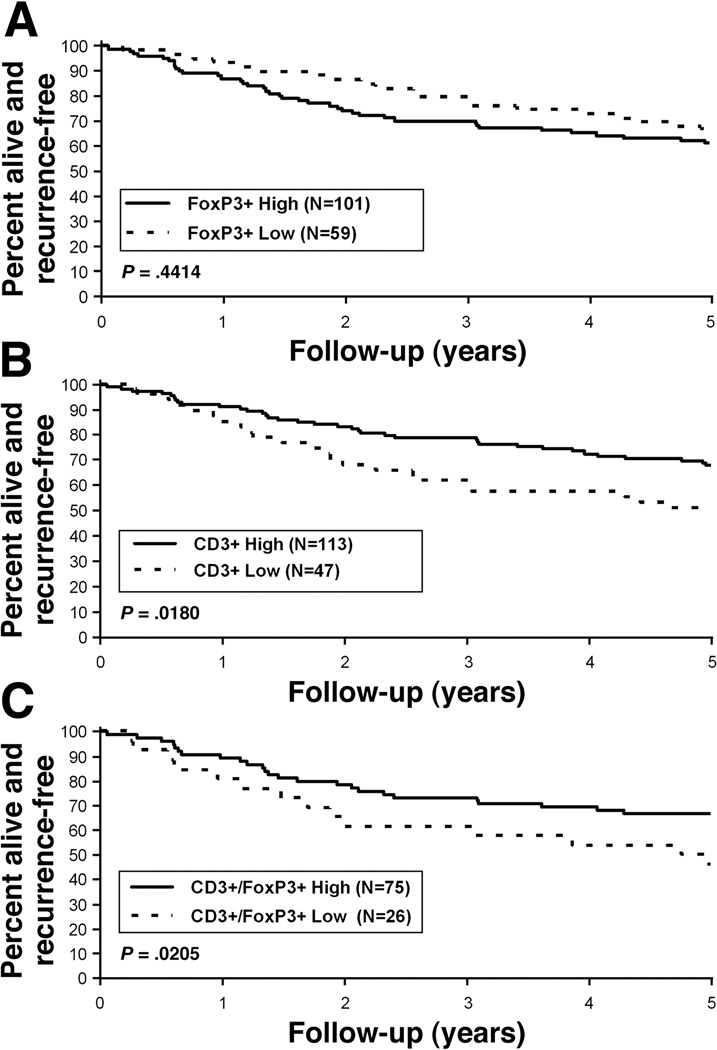
In a univariate analysis, the number of regional LN metastases was associated with significantly shorter disease-free survival (DFS) and overall survival (OS) [Table 1]. Patient age was also associated with worse OS for those 70 years or older [HR 1.75 (1.06, 2.88); p=0.0271]. The density of intraepithelial and stromal FoxP3+ and CD3+ TILs were categorized by quartile (25th percentile) and analyzed in relationship to patient survival rates. Neither the density of intraepithelial (Figure 3A) nor stromal FoxP3+ TILs was significantly associated with clinical outcome (Table 1). In contrast, low (versus higher) intraepithelial CD3+ TILs was associated with worse DFS [5-year DFS: 51.1% vs. 68.1%; HR 1.87 (1.10, 3.16); p=0.018] (Table 1, Figure 3B). Stromal CD3+ TILs, however, were not prognostic (Table 1). To further assess the contribution of FoxP3+ TILs, we analyzed the CD3+:FoxP3+ ratio and found that a low intraepithelial CD3+/FoxP3+ ratio was associated with a worse DFS as compared to a higher ratio [5-year DFS: 46.2% vs. 66.7%; HR 2.17 (1.11, 4.23); p=0.0205] (Table 1, Figure 3C). With regard to OS, the intraepithelial CD3+:FoxP3+ ratio was of borderline statistical significance [HR 1.76 (0.92, 3.37), p=0.0868)] likely due to the modest study sample size and since only 101 FoxP3+ cases were included in this ratio.
Figure 3.
Association of intraepithelial FoxP3+ (A), CD3+ (B) TILs or the CD3+:FoxP3+ ratio (C) with disease-free survival (DFS) in patients with TNM stage II and III colon carcinomas. Data are dichotomized by quartiles into high (> 1st quartile) and low (≤ 1st quartile) groups as shown.
In a multivariate analysis, a low intraepithelial CD3+:FoxP3+ ratio was independently associated with a worse DFS (HR 2.21 (1.1 – 4.45), p= 0.0318; Table 4) after adjustment for other covariates. Similarly, a low (vs. higher) density of intraepithelial CD3+ TILs was independently associated with worse DFS (HR 1.80 (1.04–3.12), p=0.0397; Table 5). Similar results were found when tumor stage (III vs II) was substituted for lymph node number in the multivariate models [CD3+:FoxP3+; HR 2.12 (1.06, 4.23), p=0.0394], [CD3+; HR 1.87 (1.09–3.21), p=0.0259]. Adjustment for MMR status in the multivariate models yielded similar hazard ratios for the CD3+:FoxP3+ ratio and CD3+ TILs alone. Also, adjustment for adjuvant treatment resulted in a similar prognostic impact for the CD3+:FoxP3+ ratio (p=0.027) and CD3+ TILs (p=0.036). Importantly, when the CD3+:FoxP3+ ratio or CD3+ T cells were included in the same multivariate model with the number of metastatic lymph nodes, only the CD3+:FoxP3+ ratio or CD3+ T cells was significant for DFS (Table 4). These results indicate that the intraepithelial CD3+:FoxP3+ ratio and the intraepithelial CD3+ T cell density are stronger prognostic variables than are the number of metastatic LNs or TNM stage.
Table 4.
Multivariate Analysis of Intraepithelial CD3+:FoxP3+ Ratio and Disease-Free Survival.
| Variable (N=101) | Hazard Ratio (95% CI)1 | P-value1 |
|---|---|---|
| CD3+/FoxP3+ TILs (≤ Quartile 1 vs. > Q1) | 2.21 (1.10, 4.45) | 0.0318 |
| Histologic Grade (3, 4 vs. 1,2)2 | 1.14 (0.57, 2.24) | 0.7151 |
| Lymph Nodes | ||
| 1–3 vs. 0 | 0.86 (0.31, 2.39) | 0.5473 |
| > 3 vs. 0 | 1.33 (0.46, 3.79) | |
| Age (≥ 70 vs. < 70) | 1.21 (0.63, 2.34) | 0.5700 |
see Methods. Results were also stratified by adjuvant study.
1,2 (well/moderate); 3,4 (poor/undifferentiated)
Table 5.
Multivariate Analysis of Intraepithelial CD3+ Density and Disease-Free Survival.
| Variable (N=160) | Hazard Ratio (95% CI)1 | P-value1 |
|---|---|---|
| CD3+ TILs (≤ Quartile 1 vs. > Q1) | 1.80 (1.04, 3.12) | 0.0397 |
| Histologic Grade (3, 4 vs. 1,2)2 | 0.99 (0.56, 1.74) | 0.9410 |
| Lymph Nodes | ||
| (1–3 vs. 0) | 1.29 (0.53, 3.14) | 0.0862 |
| (> 3 vs. 0) | 2.26 (0.91, 5.63) | |
| Age (≥ 70 vs. < 70) | 1.27 (0.74, 2.16) | 0.3928 |
see Methods. Results were also stratified by adjuvant study.
1,2 (well/moderate); 3,4 (poor/undifferentiated)
Discussion
Tregs can suppress peripheral immune responses to tumor antigens, thus maintaining immunological tolerance2, 9 and thereby, contribute to tumor progression/metastasis3, 12. We analyzed the expression and density of FoxP3+ TILs as a specific marker of Tregs,5 and CD3+ TILs as an adaptive immune marker in human colon cancers. We found that FoxP3+ TILs were significantly enriched in primary colon cancers compared to autologous normal colonic mucosa. Similar to our results in tumor tissue, Tregs in the peripheral blood of CRCs patients were shown to exceed levels found in healthy controls or from patients with inflammatory bowel disease42, 43. In patients with hepatocellular carcinoma, the prevalence of circulating FoxP3+ Tregs was increased in parallel with an accumulation of TILs in tumors, and both were associated with disease progression21. In a univariate analysis, we observed that neither the density of intraepithelial nor stromal FoxP3+ TILs was associated with clinical outcome. However, we found that patient tumors with a low epithelial CD3+:FoxP3+ ratio had a significantly worse DFS. Specifically, 46.2% of patients with a low CD3+:FoxP3+ ratio were alive and recurrence-free at 5 years compared to 66.7% for patients with a higher ratio. We also found that intraepithelial CD3+ TILs are associated with patient survival in that a lower density of intraepithelial CD3+ TILs was associated with significantly reduced DFS compared to higher CD3+ counts. The prognostic impact of CD3+ T cells underscores the importance of adaptive immunity in colon cancer prognosis28. The risk of recurrence or death, as indicated by the hazard ratios, for the CD3+:FoxP3+ ratio exceeded that of CD3+ alone in both univariate and multivariate analyses, indicating the influence of FoxP3+ TILs. The lack of prognostic impact for FoxP3+ T cells alone was similarly found in renal cell carcinomas23 and in esophageal squamous cell carcinomas;19, 24, although increased infiltrating FoxP3+ Treg have been associated with adverse outcome in certain human epithelial carcinomas12, 14, 15, 20, 21. The absence of a significant association of Tregs and prognosis in colon cancers may be related to the lack of functional assessment of Tregs in that the regulatory effect of Tregs are believed to be mainly cytokine mediated, and that FoxP3 may not universally be linked to a regulatory or suppressor phenotype in T cells23. In a multivariate analysis, we found that a low CD3+:FoxP3+ ratio as well as a lower density of intraepithelial CD3+ TILs were independent predictors of worse DFS compared to tumors with higher ratios or higher CD3+ counts. Moreover, both the CD3+:FoxP3+ ratio and CD3+ TILs were stronger prognostic variables than were TNM stage or number of metastatic LNs that are established prognostic variables in this malignancy44. This result is especially striking given the relatively modest study sample size, and suggests that the effector T cell to Treg ratio appears to be a more important variable than are Tregs alone. Since metastasis is the main event resulting in cancer-related death, our data suggest that intraepithelial CD3+ T cells and their ratio to FoxP3+ Tregs can influence tumor micrometastasis. Our findings are consistent with data in early stage non small cell lung cancer patients where a low CD3+:FoxP3+ ratio was significantly associated with adverse outcome45. Furthermore, a lower CD8+:FoxP3+ ratio was associated with adverse outcome in patients with ovarian22 and hepatocellular carcinomas46. Studies in human hepatocellular carcinoma21 and in lymphoma found that Treg cell numbers were inversely correlated with the number of cytolytic CD8+ effector T cells47. Experimental data support the clinical observations in that the ratio of Tregs to effector T cells, and not simply the presence or absence of Tregs, was the critical determinant of tumor growth in a murine sarcoma model14. Additionally, an increase in the intratumoral ratio of effector to Treg cells in poorly immunogenic murine melanomas overcame Treg-mediated suppression and tipped the balance toward tumor rejection48. These findings have therapeutic implications in that stimulation of tumor-specific effector T cells with or without Treg depletion may be a promising immunotherapeutic strategy for colon cancer. Evidence indicates that FoxP3+ Tregs in human colon cancers can inhibit tumor associated antigen-specific immune responses and that these effects can be reversed by depletion of Tregs from peripheral blood mononuclear cells42. Since the majority of patients in our study population received adjuvant chemotherapy and we have a limited sample size, we are unable to determine the predictive utility of our T cell markers for 5-FU-based therapy. However, the prognostic impact of the CD3+:FoxP3+ ratio and CD3+ T cell density were maintained after adjustment for patient treatment status. Chemotherapy has been shown to alter T cell subsets in the peripheral blood of cancer patients49 and the impact of such changes upon patient outcome will require further study.
We analyzed FoxP3+ and CD3+ T cells invading both the epithelia or stromal as their localization may potentially explain conflicting data for FoxP3+ TILs in the prognosis of epithelial cancers. We found a significantly higher density of FoxP3+ Tregs and CD3+ T cells in the tumor stroma compared with the epithelial compartment, yet the CD3:FoxP3 ratio in the epithelium exceeded that in the stroma. In a large series of CRCs, the expression level of adaptive immune markers were shown to confer prognostic information yet epithelial versus stromal localization was not distinguished28. Earlier observations indicated that the prognostic impact of T lymphocyte infiltration, including CD8+, was mainly related to the number of intraepithelial lymphocytes in CRCs22, 50, 51. In our study, the prognostic impact of the CD3+:FoxP3+ ratio and CD3+ T cells was limited to intraepithelial but not stromal infiltration, suggesting that T cells they may have distinct roles depending upon their localization. Studies in human ovarian carcinomas have shown that intraepithelial T cell expression of FoxP3,+12 CD3+52, and the CD8+/FoxP3+22 ratio were prognostic. While the explanation for the differential prognostic impact of epithelial versus stromal T cell markers is unknown, relevant factors include the following. Intraepithelial TILs are in direct contact with tumor cells and differ phenotypically and functionally from stromal lymphocytes and those in peripheral blood27, 53–55. In human CRCs, TILs in contact with tumor cells were shown to have a higher frequency of proliferation compared to stromal T cells that was observed for both CD4+ and CD8+ T cell subsets50, 56. Furthermore, FoxP3+ T cells have been shown to suppress the proliferation of other T cells that may occur by cell-to-cell contact21, in addition to cytokine-mediated regulatory effects. In this regard, interferon-γ and interleukin-2, which T cells release when activated by antigens, were readily detected in human ovarian cancers with intraepithelial T cells indicating their activation57. Most inflammatory cells, however, reside in the tumor stroma where they do not have direct contact with tumor cells in the tumor epithelium. The increased density of stromal TILs relative to epithelial FoxP3+ T cells may allow for the gradual development of immunological tolerance towards these tumors that may contribute to the lack of prognostic significance of stromal FoxP3+ or CD3+ T lymphocytes58. Interestingly, Tregs are capable of triggering high levels of functional IDO (indoleamine 2,3 dioxygenase) expression in antigen presenting cells (APC) that can anergize T cells, and high IDO expression in CRCs was associated with significant reductions of CD3+ TILs and poor patient survival59. Further studies are needed to identify the phenotype and function of Tregs and other T cell subsets in the epithelial and stromal compartments of human CRCs.
MMR deficient (vs. proficient) CRCs show an enhanced immunogenicity with increased intraepithelial lymphocytes that may contribute to their favorable clinical outcome25, 26, 30–32, 60. Studies indicate that MMR-deficient tumors can generate novel tumor-specific carboxy-terminal frameshift peptides that are recognized by tumor-infiltrating T cells61. In this study, the prognostic impact of CD3+ T cells and the CD3+:FoxP3+ ratio was maintained in the full study cohort after stratifying by tumor MMR status. We found that intraepithelial to stromal ratios for FoxP3+ and CD3+ T cells were increased in MMR-deficient vs. -proficient colon cancers, yet the CD3+:FoxP3+ ratios was similar in both groups. Intraepithelial CD8+ T cells were not significantly increased in MMR-deficient cases26. However, our sample size was limited. We found that the stromal CD8+:FoxP3+ ratio was higher in MMR-deficient vs–proficient tumors indicating an elevated effector T cell to Treg ratio. Interestingly, intraepithelial CD25+ T cells were increased in MMR-deficient tumors, whereas stromal CD25+ T cells were higher in MMR-proficient cases. This pattern was not seen for FoxP3+ Tregs, suggesting that these CD25+ effector T lymphocytes may undergo expansion and activation within the epithelia of MMR-deficient tumors. In this regard, evidence indicates that intraepithelial lymphocytes within MMR deficient tumors result from an expansion of the mucosal intraepithelial lymphocyte population rather than being peripherally derived62. Studies are needed to further characterize T cell subgroups and their functional properties based upon MMR status in a larger number of CRCs.
In summary, a low CD3+:FoxP3+ ratio and a reduced density of intraepithelial CD3+ T cells were independently associated with poorer DFS in colon cancer patients. These variables were stronger predictors of DFS than were tumor stage or number of metastatic lymph nodes, thus indicating their potential clinical utility. While these results await validation in an independent dataset, they suggest the importance of the ratio of effector T cells to Tregs in prognosis and underscore the importance of the immune microenvironment in the clinical behavior of human colon cancers.
Acknowledgments
This research was supported, in part, by National Cancer Institute (NCI) grant CA 104683-02 (to F. Sinicrope) and NCI core grant CA 15083 to the Mayo Clinic Cancer Center.
Footnotes
No conflicts of interest exist.
Author Involvement:
FA Sinicrope – Study Concept and Design, Data Analysis and Interpretation, Manuscript Writing
RL Rego – Data Acquisition, Analysis and Interpretation, Manuscript Review
SM Ansell – Acquisition of Data
KL Knutson – Data Interpretation
NR Foster – Statistical Analysis, Manuscript Review
DJ Sargent – Statistical Analysis, Manuscript Review
REFERENCES
- 1.Jemal A, Siegel R, Ward E, et al. Cancer statistics 2006. CA Cancer J Clin. 2006;56:106–130. doi: 10.3322/canjclin.56.2.106. [DOI] [PubMed] [Google Scholar]
- 2.Zou W. Regulatory T cells, tumour immunity and immunotherapy. Nat Rev Immunol. 2006;6:295–307. doi: 10.1038/nri1806. [DOI] [PubMed] [Google Scholar]
- 3.Nishikawa H, Kato T, Tawara I, et al. Accelerated chemically induced tumor development mediated by CD4+CD25+ regulatory T cells in wild-type hosts. Proc Natl Acad Sci U S A. 2005;102:9253–9257. doi: 10.1073/pnas.0503852102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shimizu J, Yamazaki S, Sakaguchi S. Induction of tumor immunity by removing CD25+CD4+ T cells: a common basis between tumor immunity and autoimmunity. J Immunol. 1999;163:5211–5218. [PubMed] [Google Scholar]
- 5.Ziegler SF. FOXP3: of mice and men. Annu Rev Immunol. 2006;24:209–226. doi: 10.1146/annurev.immunol.24.021605.090547. [DOI] [PubMed] [Google Scholar]
- 6.Hori S, Nomura T, Sakaguchi S. Control of regulatory T cell development by the transcription factor Foxp3. Science. 2003;299:1057–1061. [see comment] [PubMed] [Google Scholar]
- 7.Fontenot JD, Rudensky AY. A well adapted regulatory contrivance: regulatory T cell development and the forkhead family transcription factor Foxp3. Nature Immunology. 2005;6:331–337. doi: 10.1038/ni1179. [DOI] [PubMed] [Google Scholar]
- 8.Bensinger SJ, Walsh PT, Zhang J, et al. Distinct IL-2 receptor signaling pattern in CD4+CD25+ regulatory T cells. J Immunol. 2004;172:5287–5296. doi: 10.4049/jimmunol.172.9.5287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fontenot JD, Gavin MA, Rudensky AY. Foxp3 programs the development and function of CD4+CD25+ regulatory T cells. Nature Immunology. 2003;4:330–336. doi: 10.1038/ni904. [see comment] [DOI] [PubMed] [Google Scholar]
- 10.Yagi H, Nomura T, Nakamura K, et al. Crucial role of FOXP3 in the development and function of human CD25+CD4+ regulatory T cells. Int Immunol. 2004;16:1643–1656. doi: 10.1093/intimm/dxh165. [DOI] [PubMed] [Google Scholar]
- 11.Brunkow ME, Jeffery EW, Hjerrild KA, et al. Disruption of a new forkhead/winged-helix protein, scurfin, results in the fatal lymphoproliferative disorder of the scurfy mouse. Nat Genet. 2001;27:68–73. doi: 10.1038/83784. [DOI] [PubMed] [Google Scholar]
- 12.Curiel TJ, Coukos G, Zou L, et al. Specific recruitment of regulatory T cells in ovarian carcinoma fosters immune privilege and predicts reduced survival. Nat Med. 2004;10:942–949. doi: 10.1038/nm1093. [see comment] [DOI] [PubMed] [Google Scholar]
- 13.Turk MJ, Guevara-Patino JA, Rizzuto GA, et al. Concomitant tumor immunity to a poorly immunogenic melanoma is prevented by regulatory T cells. J Exp Med. 2004;200:771–782. doi: 10.1084/jem.20041130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wolf AM, Wolf D, Steurer M, et al. Increase of regulatory T cells in the peripheral blood of cancer patients. Clin Cancer Res. 2003;9:606–612. [PubMed] [Google Scholar]
- 15.Sasada T, Kimura M, Yoshida Y, et al. CD4+CD25+ regulatory T cells in patients with gastrointestinal malignancies: possible involvement of regulatory T cells in disease progression. Cancer. 2003;98:1089–1099. doi: 10.1002/cncr.11618. [DOI] [PubMed] [Google Scholar]
- 16.Viguier M, Lemaitre F, Verola O, et al. Foxp3 expressing CD4+CD25(high) regulatory T cells are overrepresented in human metastatic melanoma lymph nodes and inhibit the function of infiltrating T cells. J Immunol. 2004;173:1444–1453. doi: 10.4049/jimmunol.173.2.1444. [DOI] [PubMed] [Google Scholar]
- 17.Liyanage UK, Moore TT, Joo HG, et al. Prevalence of regulatory T cells is increased in peripheral blood and tumor microenvironment of patients with pancreas or breast adenocarcinoma. J Immunol. 2002;169:2756–2761. doi: 10.4049/jimmunol.169.5.2756. [DOI] [PubMed] [Google Scholar]
- 18.Yu P, Lee Y, Liu W, et al. Intratumor depletion of CD4+ cells unmasks tumor immunogenicity leading to the rejection of late-stage tumors. J Exp Med. 2005;201:779–791. doi: 10.1084/jem.20041684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wolf D, Wolf AM, Rumpold H, et al. The expression of the regulatory T cell-specific forkhead box transcription factor FoxP3 is associated with poor prognosis in ovarian cancer. Clin Cancer Res. 2005;11:8326–8331. doi: 10.1158/1078-0432.CCR-05-1244. [see comment] [DOI] [PubMed] [Google Scholar]
- 20.Bates GJ, Fox SB, Han C, et al. Quantification of regulatory T cells enables the identification of high-risk breast cancer patients and those at risk of late relapse. J Clin Oncol. 2006;24:5373–5380. doi: 10.1200/JCO.2006.05.9584. [see comment] [DOI] [PubMed] [Google Scholar]
- 21.Fu J, Xu D, Liu Z, et al. Increased regulatory T cells correlate with CD8 T-cell impairment and poor survival in hepatocellular carcinoma patients. Gastroenterology. 2007;132:2328–2339. doi: 10.1053/j.gastro.2007.03.102. [DOI] [PubMed] [Google Scholar]
- 22.Sato E, Olson SH, Ahn J, et al. Intraepithelial CD8+ tumor-infiltrating lymphocytes and a high CD8+/regulatory T cell ratio are associated with favorable prognosis in ovarian cancer. Proc Natl Acad Sci U S A. 2005;102:18538–18543. doi: 10.1073/pnas.0509182102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Siddiqui SA, Frigola X, Bonne-Annee S, et al. Tumor-infiltrating Foxp3-CD4+CD25+ T cells predict poor survival in renal cell carcinoma. Clin Cancer Res. 2007;13:2075–2081. doi: 10.1158/1078-0432.CCR-06-2139. [DOI] [PubMed] [Google Scholar]
- 24.Yoshioka T, Miyamoto M, Cho Y, et al. Infiltrating regulatory T cell numbers is not a factor to predict patient’s survival in oesophageal squamous cell carcinoma. Br J Cancer. 2008;98:1258–1263. doi: 10.1038/sj.bjc.6604294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sinicrope FA, Rego RL, Garrity-Park MM, et al. Alterations in cell proliferation and apoptosis in colon cancers with microsatellite instability. Int J Cancer. 2007;120:1232–1238. doi: 10.1002/ijc.22429. [DOI] [PubMed] [Google Scholar]
- 26.Guidoboni M, Gafa R, Viel A, et al. Microsatellite instability and high content of activated cytotoxic lymphocytes identify colon cancer patients with a favorable prognosis. Am J Pathol. 2001;159:297–304. doi: 10.1016/S0002-9440(10)61695-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Baker K, Zlobec I, Tornillo L, et al. Differential significance of tumour infiltrating lymphocytes in sporadic mismatch repair deficient versus proficient colorectal cancers: a potential role for dysregulation of the transforming growth factor-beta pathway. Eur J Cancer. 2007;43:624–631. doi: 10.1016/j.ejca.2006.11.012. [DOI] [PubMed] [Google Scholar]
- 28.Galon J, Costes A, Sanchez-Cabo F, et al. Type density, and location of immune cells within human colorectal tumors predict clinical outcome. Science. 2006;313:1960–1964. doi: 10.1126/science.1129139. [DOI] [PubMed] [Google Scholar]
- 29.Ionov Y, Peinado MA, Malkhosyan S, et al. Ubiquitous somatic mutations in simple repeated sequences reveal a new mechanism for colonic carcinogenesis. Nature. 1993;363:558–561. doi: 10.1038/363558a0. [DOI] [PubMed] [Google Scholar]
- 30.Michael-Robinson JM, Biemer-Huttmann A, Purdie DM, et al. Tumour infiltrating lymphocytes and apoptosis are independent features in colorectal cancer stratified according to microsatellite instability status. Gut. 2001;48:360–366. doi: 10.1136/gut.48.3.360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Popat S, Hubner R, Houlston RS. Systematic review of microsatellite instability and colorectal cancer prognosis. J Clin Oncol. 2005;23:609–618. doi: 10.1200/JCO.2005.01.086. [DOI] [PubMed] [Google Scholar]
- 32.Sinicrope FA, Rego RL, Halling KC, et al. Prognostic impact of microsatellite instability and DNA ploidy in human colon carcinoma patients. Gastroenterology. 2006;131:729–737. doi: 10.1053/j.gastro.2006.06.005. [DOI] [PubMed] [Google Scholar]
- 33.Allegra CJ, Parr AL, Wold LE, et al. Investigation of the prognostic and predictive value of thymidylate synthase p53, and Ki-67 in patients with locally advanced colon cancer. J Clin Oncol. 2002;20:1735–1743. doi: 10.1200/JCO.2002.07.080. [DOI] [PubMed] [Google Scholar]
- 34.O'Connell MJ, Sargent DJ, Windschitl HE, et al. Randomized clinical trial of high-dose levamisole combined with 5-fluorouracil and leucovorin as surgical adjuvant therapy for high-risk colon cancer. Clinical Colorectal Cancer. 2006;6:133–139. doi: 10.3816/ccc.2006.n.030. [DOI] [PubMed] [Google Scholar]
- 35.Halling KC, French AJ, McDonnell SK, et al. Microsatellite instability and 8p allelic imbalance in stage B2 and C colorectal cancers. J Natl Cancer Inst. 1999;91:1295–1303. doi: 10.1093/jnci/91.15.1295. [see comment] [DOI] [PubMed] [Google Scholar]
- 36.Thibodeau SN, French AJ, Cunningham JM, et al. Microsatellite instability in colorectal cancer: different mutator phenotypes and the principal involvement of hMLH1. Cancer Res. 1998;58:1713–1718. [PubMed] [Google Scholar]
- 37.de la Chapelle A. Testing tumors for microsatellite instability. Eur J Hum Genet. 1999;7:407–408. doi: 10.1038/sj.ejhg.5200335. [DOI] [PubMed] [Google Scholar]
- 38.Bluestone JA, Abbas AK. Natural versus adaptive regulatory T cells. Nat Rev Immunol. 2003;3:253–257. doi: 10.1038/nri1032. [DOI] [PubMed] [Google Scholar]
- 39.Weisenberger DJ, Siegmund KD, Campan M, et al. CpG island methylator phenotype underlies sporadic microsatellite instability and is tightly associated with BRAF mutation in colorectal cancer. Nat Genet. 2006;38:787–793. doi: 10.1038/ng1834. [DOI] [PubMed] [Google Scholar]
- 40.Aaltonen LA, Salovaara R, Kristo P, et al. Incidence of hereditary nonpolyposis colorectal cancer and the feasibility of molecular screening for the disease. N Engl J Med. 1998;338:1481–1487. doi: 10.1056/NEJM199805213382101. [DOI] [PubMed] [Google Scholar]
- 41.Cao X, Cai SF, Fehniger TA, et al. Granzyme B and perforin are important for regulatory T cell-mediated suppression of tumor clearance. Immunity. 2007;27:635–646. doi: 10.1016/j.immuni.2007.08.014. [DOI] [PubMed] [Google Scholar]
- 42.Clarke SL, Betts GJ, Plant A, et al. CD4+CD25+FOXP3+ Regulatory T Cells Suppress Anti-Tumor Immune Responses in Patients with Colorectal Cancer. PLoS ONE. 2006;1:e129. [Google Scholar]
- 43.Ling KL, Pratap SE, Bates GJ, et al. Increased frequency of regulatory T cells in peripheral blood and tumour infiltrating lymphocytes in colorectal cancer patients. Cancer Immunity. 2007;7:7. [PMC free article] [PubMed] [Google Scholar]
- 44.O'Connell JB, Maggard MA, Ko CY. Colon cancer survival rates with the new American Joint Committee on Cancer sixth edition staging. J Natl Cancer Inst. 2004;96:1420–1425. doi: 10.1093/jnci/djh275. [DOI] [PubMed] [Google Scholar]
- 45.Petersen RP, Campa MJ, Sperlazza J, et al. Tumor infiltrating Foxp3+ regulatory T-cells are associated with recurrence in pathologic stage I NSCLC patients. Cancer. 2006;107:2866–2872. doi: 10.1002/cncr.22282. [DOI] [PubMed] [Google Scholar]
- 46.Gao Q, Qiu SJ, Fan J, et al. Intratumoral balance of regulatory and cytotoxic T cells is associated with prognosis of hepatocellular carcinoma after resection. J Clin Oncol. 2007;25:2586–2593. doi: 10.1200/JCO.2006.09.4565. [DOI] [PubMed] [Google Scholar]
- 47.Yang ZZ, Novak AJ, Ziesmer SC, et al. Attenuation of CD8(+) T-cell function by CD4(+)CD25(+) regulatory T cells in B-cell non-Hodgkin's lymphoma. Cancer Res. 2006;66:10145–10152. doi: 10.1158/0008-5472.CAN-06-1822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Quezada SA, Peggs KS, Curran MA, et al. CTLA4 blockade and GM-CSF combination immunotherapy alters the intratumor balance of effector and regulatory T cells. J Clin Invest. 2006;116:1935–1945. doi: 10.1172/JCI27745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Petrini B, Wasserman J, Blomgren H, et al. Changes of blood T cell subsets in patients receiving postoperative adjuvant chemotherapy for breast cancer. Eur J Cancer Clin Oncol. 1984;20:1485–1487. doi: 10.1016/0277-5379(84)90141-x. [DOI] [PubMed] [Google Scholar]
- 50.Naito Y, Saito K, Shiiba K, et al. CD8+ T cells infiltrated within cancer cell nests as a prognostic factor in human colorectal cancer. Cancer Res. 1998;58:3491–3494. [PubMed] [Google Scholar]
- 51.Chiba T, Ohtani H, Mizoi T, et al. Intraepithelial CD8+ T-cell-count becomes a prognostic factor after a longer follow-up period in human colorectal carcinoma: possible association with suppression of micrometastasis. Br J Cancer. 2004;91:1711–1717. doi: 10.1038/sj.bjc.6602201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Tomsova M, Melichar B, Sedlakova I, et al. Prognostic significance of CD3+ tumor-infiltrating lymphocytes in ovarian carcinoma. Gynecol Oncol. 2008;108:415–420. doi: 10.1016/j.ygyno.2007.10.016. [DOI] [PubMed] [Google Scholar]
- 53.Takemoto N, Konishi F, Yamashita K, et al. The correlation of microsatellite instability and tumor-infiltrating lymphocytes in hereditary non-polyposis colorectal cancer (HNPCC) and sporadic colorectal cancers: the significance of different types of lymphocyte infiltration. Jpn J Clin Oncol. 2004;34:90–98. doi: 10.1093/jjco/hyh018. [DOI] [PubMed] [Google Scholar]
- 54.Quinn E, Hawkins N, Yip YL, et al. CD103+ intraepithelial lymphocytes--a unique population in microsatellite unstable sporadic colorectal cancer. Eur J Cancer. 2003;39:469–475. doi: 10.1016/s0959-8049(02)00633-0. [DOI] [PubMed] [Google Scholar]
- 55.Ebert EC, Roberts AI. Lymphokine-activated killing by human intestinal lymphocytes. Cell Immunol. 1993;146:107–116. doi: 10.1006/cimm.1993.1010. [DOI] [PubMed] [Google Scholar]
- 56.Golby SJ, Chinyama C, Spencer J. Proliferation of T-cell subsets that contact tumour cells in colorectal cancer. Clin Exp Immunol. 2002;127:85–91. doi: 10.1046/j.1365-2249.2002.01730.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zhang L, Conejo-Garcia JR, Katsaros D, et al. Intratumoral T cells recurrence, and survival in epithelial ovarian cancer. N Engl J Med. 2003;348:203–213. doi: 10.1056/NEJMoa020177. [DOI] [PubMed] [Google Scholar]
- 58.Reiman JM, Kmieciak M, Manjili MH, et al. Tumor immunoediting and immunosculpting pathways to cancer progression. Semin Cancer Biol. 2007;17:275–287. doi: 10.1016/j.semcancer.2007.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Brandacher G, Perathoner A, Ladurner R, et al. Prognostic value of indoleamine 2,3-dioxygenase expression in colorectal cancer: effect on tumor-infiltrating T cells. Clin Cancer Res. 2006;12:1144–1151. doi: 10.1158/1078-0432.CCR-05-1966. [DOI] [PubMed] [Google Scholar]
- 60.Jass JR, Do KA, Simms LA, et al. Morphology of sporadic colorectal cancer with DNA replication errors. Gut. 1998;42:673–679. doi: 10.1136/gut.42.5.673. [see comment] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Schwitalle Y, Kloor M, Eiermann S, et al. Immune response against frameshift-induced neopeptides in HNPCC patients and healthy HNPCC mutation carriers. Gastroenterology. 2008;134:988–997. doi: 10.1053/j.gastro.2008.01.015. [DOI] [PubMed] [Google Scholar]
- 62.Baker K, Foulkes WD, Jass JR. MSI-H colorectal cancers preferentially retain and expand intraepithelial lymphocytes rather than peripherally derived CD8+ T cells. Cancer Immunol Immunother. 2009;58:135–144. doi: 10.1007/s00262-008-0534-1. [DOI] [PMC free article] [PubMed] [Google Scholar]