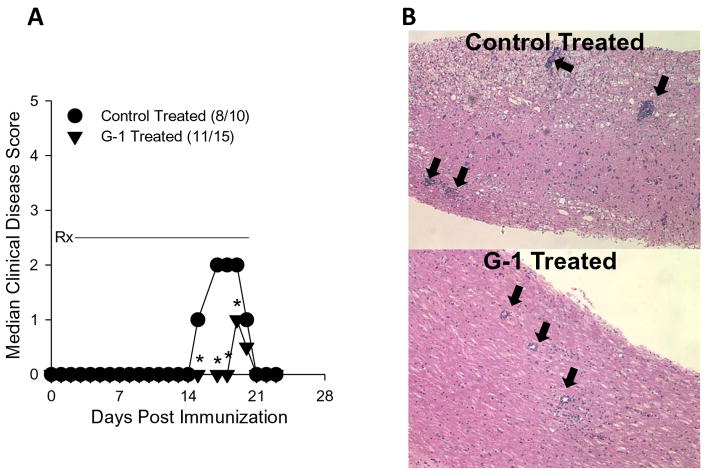
Figure 5.
GPR30 agonist treatment reduced the severity of actively induced experimental autoimmune encephalomyelitis. A) SJL mice (5–7 weeks old) were immunized s.c. with 50 μg PLP139-151 and CFA (400 μg Mycobacterium tuberculosis). Mice were treated with 50 mg/kg/day G-1 daily for 21 days beginning at the day of disease induction (Rx). Control mice were similarly treated with vehicle (5% DMSO, 95% PEG-300). Clinical disease was scored as 0, asymptomatic; 1, tail atonia; 2, hind limb paresis; 2, unilateral hind limb paralysis; 4, bilateral hind limb paralysis; and 5, death. The data are shown as the median clinical disease score as a function of days post immunization. Disease incidence for each treatment group is indicated in parentheses. The G-1-treated group showed significantly decreased acute clinical disease score, but not incidence, compared to control mice (*, p<0.05). B) The CNS of representative mice from each treatment group was evaluated for histologic EAE by standard H&E staining. The photomicrographs (100x magnification) indicate less severe mononuclear cell lesions in the G-1- compared to control-treated mice (heavy black arrows). These data are representative of three similar, independent experimental replicates.