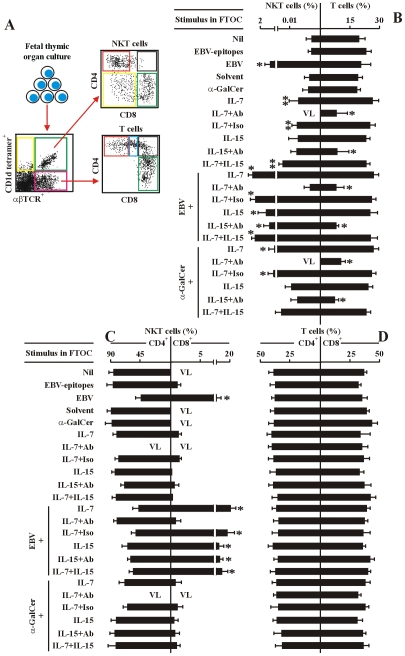
Figure 5. IL-7 is required for EBV-induced CD8+ NKT cell differentiation in vitro.
(A) The experimental and analysis scheme for detecting co-receptor-expressing NKT cells and T cells in various human fetal thymic organ cultures. After 14-day-culture, the various cell types were identified by flow cytometry as in Figure 2. (B–D) Frequency of total NKT cells and total T cells (B), co-receptor-expressing NKT cells (C) and T cells (D) in the different FTOCs. The protocols for the establishment of different FTOCs were described in the leftmost panels in each sub-figure. The various stimuli and blockers were added as indicated. Nil, no stimulus; EBV-epitopes, HLA-A2-restricted, derived from the lytic cycle protein BMLF1 and HLA-DRB1-restricted, derived from nuclear antigen EBNA1 (1 µg/ml each); EBV, infectious EBV (107 pfu); Solvent, 0.005% polysorbate 20; α-GalCer (0.1 µg/ml). IL-7 (10 ng/ml); IL-15 (10 ng/ml), Abs, either mouse anti-human IL-7 monoclonal Ab plus mouse anti-human IL-7Rα monoclonal Ab, or mouse anti-human IL-15 monoclonal Ab plus mouse anti-human IL-15Rα monoclonal Ab, respectively (5 µg/ml each); The FTOCs were harvested and assessed by flow cytometry. Only the data for CD4+ and CD8+ NKT cells and CD4 SP and CD8 SP T cells were shown. VL, very low level (below detectable level). Data were mean ± s.d. (n = 10). ** p<0.05. *, p<0.001, EBV-challenged FTOCs vs. non-challenged FTOCs; IL-7-challenged FTOCs vs. non-challenged FTOCs.