Abstract
Current methods for determining and dissecting the function of a specific protein within a cell are laborious and limiting. We have developed a method by which endogenous protein levels are rapidly ablated and simultaneous expression of a designed, inserted variant takes place in the native setting. Through optimized electroporation, siRNA oligonucleotides and codon-optimized coding sequence containing vectors can be co-transfected, leading to expression of ectopic mRNA not targeted by siRNA. Using the commonly encountered MCF-7 breast cancer cell line, we were able to reach 90% transfection efficiency. Under these conditions, siRNA oligonucleotides were transfected simultaneously with a codon-optimized, cDNA containing vector encoding the AHR protein. Thus, endogenous protein was ablated while the designed protein was fully expressed in the native environment. The codon-optimized AHR was shown to be fully functional in its ability to induce CYP1A1 transcription and to rescue a B[a]P-susceptible phenotype.
Keywords: Electroporation, Ectopic expression, siRNA, Transfection, AHR, AH receptor, MCF-7
Introduction
The analysis of protein function is of great utility in understanding cellular responses and intrinsic activities. While numerous computational and experimental approaches exist to tease out the functional domains of a protein, as well as the larger role that a protein may play in a signaling pathway, there is a lack of methods through which protein function can truly be assessed within the native environment in a variety of cell lines.
In order to analyze the effects of a protein in its cellular environment, two basic approaches are available. One approach would be to introduce protein constructs into null cells, while the other approach involves ablation of a specific protein from cells in which it is normally present. Both methods have their disadvantages in terms of the environment being analyzed and the techniques utilized. Traditionally, the first approach used to assess the role that a given protein exhibits in a cellular pathway utilizes either a cell type that does not express that protein, or cells derived from a null mouse. Examining protein function within the context of human cells is even more difficult, considering that null cell types are not available. Protein expression is usually achieved in these null cells through the generation of a cell line with a stably integrated plasmid or through the use of high efficiency viral delivery systems. Often, expression of mutant forms of the protein of interest is used to dissect its function. Each system has its disadvantages, including perturbation of normal cellular pathways, not to mention the time and safety issues associated with creating a stable cell line and working with viral systems. In some cell lines (e.g. COS 1), highly efficient expression can be transiently obtained using chemical transfection reagents. However, these reagents have unknown effects on cells through changes in plasma membrane integrity and function, induction of Ca2+ mediated signaling, and can be toxic. The second approach commonly used to study protein function is to ablate expression of a specific protein through the use of siRNA or shRNA vector or viral delivery systems, allowing for the examination of appropriate endpoints in the absence of the protein of interest. This approach suffers from some of the same limitations as those described above. An ideal approach would combine the methods of creating a null protein environment and expression of a protein construct, and would be applicable to a wide range of cells. This approach would also allow the dissection of protein function through the ablation of endogenous protein expression and the simultaneous expression of various mutants.
An ideal approach would combine the methods of creating a null protein environment and expression of a protein construct in the absence of chemical transfection reagents, and would be applicable to a wide range of cells. One answer to this problem was in part addressed by the method of Wu et al. (2006), in which native proteins are ablated via siRNA and constructs are simultaneously expressed that escape siRNA-mediated turnover. However, the use of chemical transfection limits the cell types available for study due to sub-optimal efficiency in most lines. Another issue with their results is the fact that, without near total ablation of endogenous protein, the inserted construct is not the only source to contribute to the functional endpoints analyzed.
Our laboratory is engaged in research that centers on the aryl hydrocarbon receptor (AHR); a cytosolic protein that binds ligands and acts as a transcription factor via DNA binding. The AHR exhibits diverse cellular activities, the most studied of which concerns its ability to regulate a number of genes involved in xenobiotic metabolism (Beischlag et al. 2008). More recently, the AHR has been shown to regulate certain aspects of inflammatory signaling such as T cell differentiation, acute phase response and IL6 expression in tumor cells (Veldhoen et al. 2009; Patel et al. 2009; Hollingshead et al. 2008). Some of these effects are seen to occur at different levels across cell types. Therefore, a full understanding of the multiple pathways and activities of the AHR would require the ability to study various mutant versions of the receptor in the native environment across different cell types. We have designed a protocol that allows for rapid and efficient expression of an ectopic protein in place of endogenous protein in the native environment, without the use of chemical transfection agents or viral constructs that can obscure results.
Materials and methods
Cell culture
MCF-7 breast tumor cells were maintained at 37°C, 5% CO2 in a high glucose DMEM (Sigma), supplemented with 7% fetal bovine serum (FBS; Hyclone Labs.), 1,000 units/mL penicillin, and 0.1 mg/mL streptomycin (Sigma).
Transfection
Cells were transfected via electroporation using Amaxa’s nucleofector II system, essentially as per manufacturer protocol. Briefly, cells were washed and suspended at a concentration of 2.0 × 106 cells per 100 μL of room temperature electroporation solution (272 mM sucrose, 1 mM MgCl2 in PBS, sterile-filtered). siRNA oligonucleotides were added at a final concentration of 1.5 μM and DNA vector was added at a final concentration of 10 μg/mL. Suspension was then pipetted into a reaction cuvette and electroporated using the manufacturer’s MCF-7 High Efficiency protocol. Room temperature medium was immediately added to cuvette following electroporation and cells were subsequently plated into six-well dishes of complete medium.
siRNA and vectors
Electroporation of green fluorescent protein vector was carried out using the pmax GFP from Amaxa. AHR protein levels were decreased using the Dharmacon small interfering RNA (siRNA) [J004990-07], Dharmacon off target siRNA [D001210-02] was used for control electroporation. The vector pcDNA3 was used without change as the control vector, or was digested and ligated with a synthetic codon-optimized AHR sequence produced by GenScript. Homology analysis of the relevant endogenous hAHR cDNA and synthetic codon-optimized hAHR cDNA sequences, as well as the siRNA targeting oligonucleotide is shown below
| Endogenous hAHR cDNA | GCACGAGAGGCTCAGGTTA |
| Bases 947: 965 | |
| Synthetic codon-optimized hAHR cDNA | GCACACGGGGCAGCGGCTA |
| Bases 947: 965 | |
| hAHR targeting siRNA | GCACGAGAGGCUCAGGUUA |
Cell imaging
Cells were imaged on a Nikon Eclipse TE300 inverted epi-fluorescence microscope, using a Nikon mercury arc lamp and filter set for fluorescence. Images were taken using a Roper Scientific CoolSnap ES CCD camera system and collected using the MetaVue software package. All images shown are at a 10× objective.
Immunoblotting
Whole-cell extracts were prepared by lysing cells in 1× radioimmunoprecipitation assay buffer [RIPA; 10 mmol/L Tris–HCl (pH 8.0), 1 mmol/L EDTA, 0.5 mmol/L EGTA, 140 mmol/L NaCl, 1% Triton X-100, 0.1% Na-deoxycholate, 0.1% SDS] supplemented with 1% NP40, 300 mM NaCl, and protease inhibitor cocktail (Sigma). Homogenates were centrifuged at 21,000×g for 30 min at 4°C, and the soluble fraction was collected as whole-cell extract. Protein concentrations were determined using the detergent compatible Bio-Rad DC protein assay kit (Bio-Rad).
Protein samples were resolved by Tricine SDS–PAGE and transferred to membrane. Immunoblotting was performed using antibodies directed against AHR and p23 (provided by Dr. David Toft, Mayo Clinic). Proteins were visualized using biotin-conjugated secondary antibodies (Jackson Immunoresearch) in conjunction with I125 streptavidin.
Gene expression
MCF-7 cells were serum-starved 18 h before experimentation. Treatment of cells was performed by diluting 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) to 1 nM in serum-free medium supplemented with 5 mg/mL bovine serum albumin (BSA) or adding an equivalent volume of DMSO for control treatment. Cells were treated for 2 h, washed, and total RNA was extracted using TRI reagent (Sigma) as specified by the manufacturer. The ABI high-capacity cDNA archive kit (Applied Biosystems) was used to prepare cDNA from isolated RNA. Measurements of mRNA expression for all samples were performed by quantitative real-time PCR using the Quanta PerfeCTa SYBR Green Supermix kit on an iCycler DNA engine equipped with the MyiQ single color real-time PCR detection system (Bio-Rad). Expressed quantities of mRNA were normalized to GAPDH levels and plotted using GraphPad Prism 4.00 (GraphPad Software). Data are plotted as mean values of triplicate biological samples, and error bars represent the SDs.
Benzo[a]pyrene (B[a]P) toxicity assay
MCF-7 cells were washed, counted, transfected, and plated at 30% confluency in six-well dishes of complete medium for 24 h. Cells were then washed and medium was changed to serum-free medium supplemented with 5 mg/mL BSA and containing DMSO (control), or 10 μM B[a]P. Images were taken at the time of media change (Time 0) and 96 h later. Quantification represents cell counts across 4 samples of field of view and biological replicates.
Results and discussion
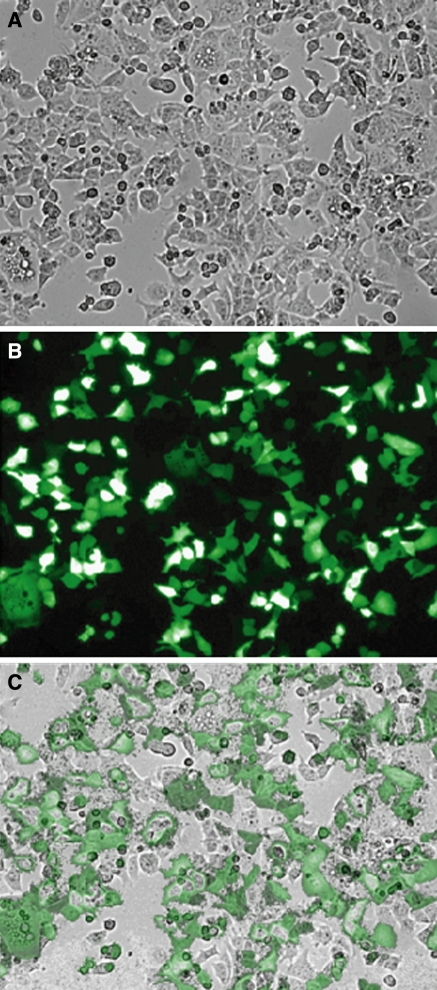
We began by optimizing transfection rates for MCF-7 cells using the Amaxa nucleofector electroporation device to obtain roughly 90% transfection efficiency (Fig. 1). Obtaining greater than 90% efficiency in a cell type requires optimization of variables such as cell density, ionic levels in electroporation solution, DNA/siRNA concentration, and electric field generation (Jordan et al. 2008; Klenchin et al. 1991; Sukharev et al. 1992). Optimization of transfection efficiency must be balanced with cell viability following electroporation, a factor that is also cell type-specific. Electroporation of MCF-7 cells under our conditions results in roughly 50% cell viability, which is not uncommon for electroporation (Kang et al. 2009). Near complete efficiency usually leads to low viability, which is easily overcome with the use of an appropriate number of cells.
Fig. 1.
Optimized electroporation allows for high rates of vector transfection. MCF-7 cells were electroporated with a GFP vector using the described protocol. a Brightfield image of cells 48 h post-transfection. b Fluorescence image of GFP expressing cells 48 h post-transfection. c Overlay of figures (a) and (b) shows a high rate of transfection of the vector
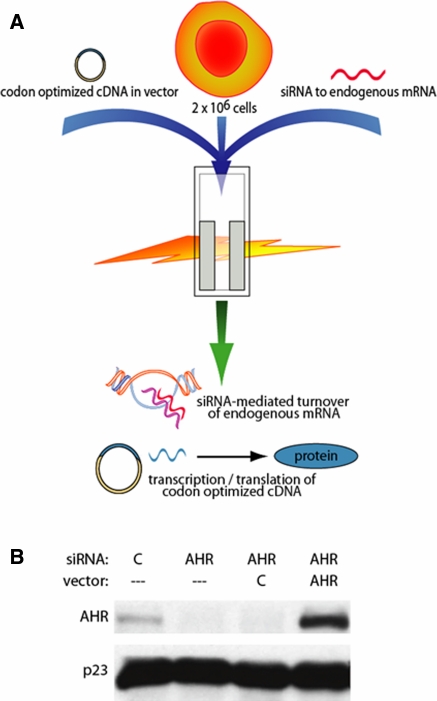
Upon realizing highly efficient transfection in the MCF-7 cell line, dual transfections were performed with siRNA oligonucleotides and codon-optimized cDNA-containing vectors. While siRNA oligonucleotides target mRNA and reduce endogenous protein levels, simultaneous transcription of a promoter-containing vector with a codon optimized coding sequence expressing an mRNA transcript that is not targeted by the same siRNA occurs (Fig. 2a). Codon-optimized cDNA sequences can be commercially synthesized by a company such as GenScript, or site-directed mutagenesis can be carried out to codon optimize the stretch of cDNA targeted by the siRNA. Both methods allow for further subsequent alteration to create mutant proteins. We transfected MCF-7 cells with a control siRNA, an AHR targeted siRNA, a targeted siRNA and control vector, or a targeted siRNA and a vector containing a codon-optimized cDNA that encodes wild-type human AHR. The high efficiency of electroporation with the targeted siRNA led to a near complete ablation of endogenous protein levels 48 h after transfection, and the co-transfection with the control vector showed minimal change in protein knockdown. Therefore, the AHR protein shown following co-transfection has originated from the introduced vector (Fig. 2b). Due to the increased efficiency of transcription with respect to the codon-optimized vector, AHR protein levels are higher following transfection as compared to endogenous protein levels.
Fig. 2.
Simultaneous ablation of endogenous protein and expression of designed ectopic protein. a Cells are suspended with a protein-targeting siRNA oligonucleotide and a codon-optimized DNA-containing vector, electroporated, and plated. In 24 to 48 h, the siRNA ablates endogenous protein, while the vector is transcribed to an mRNA which encodes the same protein but is not targeted by the siRNA. b MCF-7 cells were electroporated as indicated in figure key. Western blot showing AHR protein levels and the hsp90 cochaperone p23 was used as a loading control 48 h after transfection. Vector C is the control vector pcDNA3
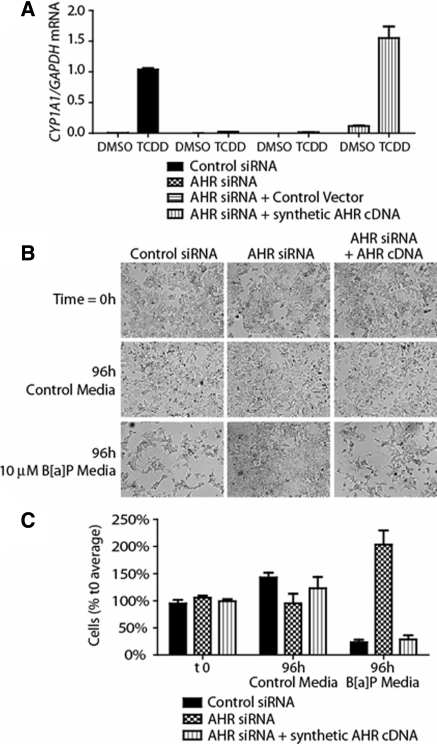
AHR activity greatly increases transcription of the cytochrome P450 1A1 (CYP1A1) gene (Dong et al. 1996; Whitlock 1999). Following co-transfection, cells were treated with the AHR ligand TCDD for 2 h, after which RNA was isolated and subjected to quantitative RT-PCR to determine CYP1A1 mRNA levels. In accordance with AHR protein levels, ligand treatment led to CYP1A1 induction in control transfection cells, while AHR knockdown with or without the control vector ablated this activity. Co-transfection with the AHR expressing vector regained CYP1A1 induction at higher levels, in parallel with the higher protein expression (Fig. 3a). Greater AHR protein expression following transfection leads to a slight increase in basal CYP1A1 transcription as well as an increase in transcription following exposure to a non-saturating dose of TCDD. We have additionally used this method to co-transfect MCF-7 cells with an altered, codon-optimized cDNA vector that encodes an AHR DNA-binding mutant, which showed the expected outcome when it failed to significantly induce CYP1A1 activity (data not shown).
Fig. 3.
Co-transfection of siRNA and codon-optimized cDNA-containing vector restores wild-type target gene expression and cellular sensitivity to B[a]P exposure. MCF-7 cells were electroporated as indicated in figure key. a Quantitative real-time PCR of transfected cells for CYP1A1 mRNA, normalized to GAPDH mRNA. b Inverted microscope images showing MCF-7 viability following B[a]P exposure. c Quantification of cell viability following 96 h exposure to control or B[a]P-containing media. Cell counts are normalized to the average of cells plated at t 0
Long-term exposure to AHR ligands including benzo-a-pyrene (B[a]P) has been shown to lead to cell death through AHR-mediated B[a]P metabolism to reactive intermediates (Park et al. 1996; Hankinson 1979). To utilize this concept in our system, MCF-7 cells were transfected with control siRNA, AHR targeted siRNA, or co-transfected with an AHR siRNA and the codon-optimized cDNA-containing vector. Transfected cells were then plated in either control media or media containing 10 μM B[a]P for 96 h. Cells were imaged at a 0 time point when treated medium was added, and following 96 h of culture time. Long-term B[a]P treatment led to a drastic reduction in the number of viable control transfection cells, while AHR knockdown cells were able to remain viable and proliferate in the presence of B[a]P. Introduction of the codon-optimized AHR vector rescued the phenotype of control cells; namely, vulnerability to long-term B[a]P exposure (Fig. 3b, c).
This method for rapid, transient expression of wild-type or mutant proteins in their native environment can potentially be used in any cell line following transfection optimization. In creating this protocol, we aimed to expand the abilities of researchers to study protein interactions and pathways with much less effort. A rapid method of transient expression of ectopic proteins can be indispensable in examining protein function through mutated proteins, under/overexpression of proteins, etc. Upon optimization of electroporation methods for a specific cell type, simple co-transfection with a targeted siRNA oligonucleotide and a codon-optimized DNA-containing vector can allow for transient expression of a mutant protein in its native environment within 24 to 48 h.
Acknowledgments
Supported by Public Health Service grant ES04869 from the National Institute of Environmental Health Sciences.
References
- Beischlag TV, Morales JL, Hollingshead BD, Perdew GH. The aryl hydrocarbon receptor complex and the control of gene expression. Crit Rev Eukaryot Gene Expr. 2008;18:207–250. doi: 10.1615/critreveukargeneexpr.v18.i3.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong L, Ma Q, Whitlock JP., Jr DNA binding by the heterodimeric Ah receptor. Relationship to dioxin-induced CYP1A1 transcription in vivo. J Biol Chem. 1996;271:7942–7948. doi: 10.1074/jbc.271.14.7942. [DOI] [PubMed] [Google Scholar]
- Hankinson O. Single-step selection of clones of a mouse hepatoma line deficient in aryl hydrocarbon hydroxylase. Proc Natl Acad Sci USA. 1979;76:373–376. doi: 10.1073/pnas.76.1.373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollingshead BD, Beischlag TV, DiNatale BC, Ramadoss P, Perdew GH. Inflammatory signaling and aryl hydrocarbon receptor mediate synergistic induction of interleukin 6 in MCF-7 cells. Cancer Res. 2008;68:3609–3617. doi: 10.1158/0008-5472.CAN-07-6168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jordan ET, Collins M, Terefe J, Ugozzoli L, Rubio T. Optimizing electroporation conditions in primary and other difficult-to-transfect cells. J Biomol Tech. 2008;19:328–334. [PMC free article] [PubMed] [Google Scholar]
- Kang J, Ramu S, Lee S, Aguilar B, Ganesan SK, Yoo J, Kalra VK, Koh CJ, Hong YK. Phosphate-buffered saline-based nucleofection of primary endothelial cells. Anal Biochem. 2009;15;386:251–255. doi: 10.1016/j.ab.2008.12.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klenchin VA, Sukharev SI, Serov SM, Chernomordik LV, Chizmadzhev YuA. Electrically induced DNA uptake by cells is a fast process involving DNA electrophoresis. Biophys J. 1991;60:804–811. doi: 10.1016/S0006-3495(91)82115-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park JY, Shigenaga MK, Ames BN. Induction of cytochrome P4501A1 by 2, 3, 7, 8-tetrachlorodibenzo-p-dioxin or indolo(3, 2-b)carbazole is associated with oxidative DNA damage. Proc Natl Acad USA. 1996;9:2322–2327. doi: 10.1073/pnas.93.6.2322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel RD, Murray IA, Flaveny CA, Kusnadi A, Perdew GH. Ah receptor represses acute-phase response gene expression without binding to its cognate response element. Lab Invest. 2009;89:695–707. doi: 10.1038/labinvest.2009.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sukharev SI, Klenchin VA, Serov SM, Chernomordik LV, Chizmadzhev YuA. Electroporation and electrophoretic DNA transfer into cells. The effect of DNA interaction with electropores. Biophys J. 1992;63:1320–1327. doi: 10.1016/S0006-3495(92)81709-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veldhoen M, Hirota K, Christensen J, O’Garra A, Stockinger B. Natural agonists for aryl hydrocarbon receptor in culture medium are essential for optimal differentiation of Th17 T cells. J Exp Med. 2009;206:43–49. doi: 10.1084/jem.20081438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitlock JP., Jr Induction of cytochrome P4501A1. Annu Rev Pharmacol Toxicol. 1999;39:103–125. doi: 10.1146/annurev.pharmtox.39.1.103. [DOI] [PubMed] [Google Scholar]
- Wu W, Hodges E, Höög C. Thorough validation of siRNA-induced cell death phenotypes defines new anti-apoptotic protein. Nucleic Acids Res. 2006;34:e13. doi: 10.1093/nar/gnj015. [DOI] [PMC free article] [PubMed] [Google Scholar]