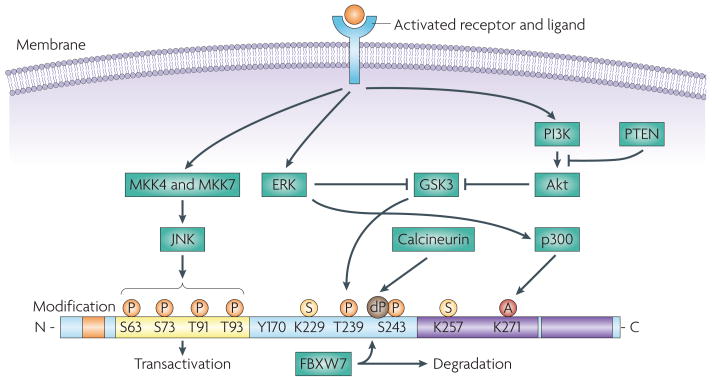
Figure 1. Structure and regulation of JUN.
JUN is encoded by a 3.34 kb intronless gene, located on chromosome 1 (1p32-p31) and results in the expression of a 334 amino acid protein product composed of four main domains, which are involved in DNA binding, transcription and dimerization197. JUN activity is regulated by post-translational modifications, which are largely controlled by components of the MAPK family of serine and threonine kinases, including JUN N-terminal kinase (JNK), ERK and p38 isoforms17. JUN is phosphorylated on Ser63 and Ser73 by JNK, increasing its stability and transactivation potential35. JNK also phosphorylates Thr91 and Thr93, which are required for DNA binding and activation of its transcriptional activity. JUN is subject to ubiquitylation and this requires phosphorylation at Thr239 by glycogen synthase kinase 3 (GSK3). GSK3 can target JUN only once Ser243 is phosphorylated. Phosphorylation on these sites is required for recruitment of the F-box and WD domain repeated 7 (FBXW7) ubiquitin ligase198. Inactivation of GSK3, owing to the activation of ERK and PI3K–Akt signalling cascades results in JUN stabilization44,45. The effect of GSK3 can be antagonized by the dephosphorylation of Ser243 by calcineurin199. JUN can be sumoylated on Lys257 and Lys229, which leads to a reduced transcriptional activity51. ERK induces the acetylation of the lysine residues in the JUN DNA binding region200, thereby increasing JUN transcriptional activity. Post-translational modifications are indicated as small coloured circles. The four domains are indicated as follows: the δ-domain is orange, the basic region (DNA binding) is blue, the transactivation domain is yellow and the leucine zipper is purple. A, acetylation; dP, dephosphorylation; P, phosphorylation; S, sumoylation.