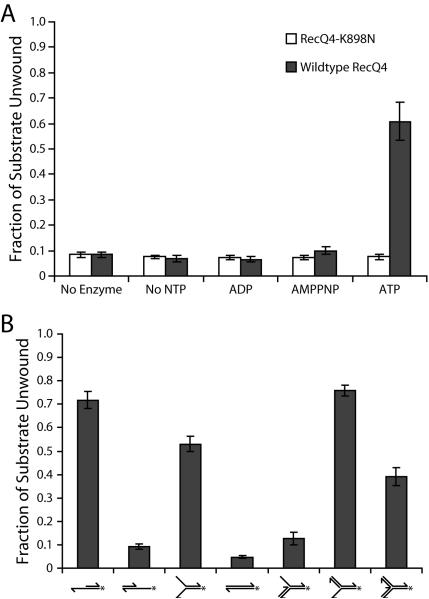
Figure 4.
Analysis of the helicase activity substrate preference of Drosophila RecQ4. (A) RecQ4 (shaded bars) unwinds the 3' extension substrate in the presence of ATP. This activity is due to the helicase domain of RecQ4 because the helicase inactive mutant RecQ4-K898N (open bars) does not unwind the 3' extension substrate. (B) Quantification of RecQ4 activity on substrates. RecQ4 acts as a helicase only on substrates with a 3' single-stranded region, with the exception of the 3-way duplex junction substrate (far right). Error bars in these experiments indicate standard deviation (n =3). This research was originally published in the Journal of Biological Chemistry. Capp et al. Drosophila RecQ4 Has a 3'-5' DNA Helicase Activity That Is Essential for Viability. J. Biol. Chem. 2009; 284:30845–30852. © the American Society for Biochemistry and Molecular Biology.