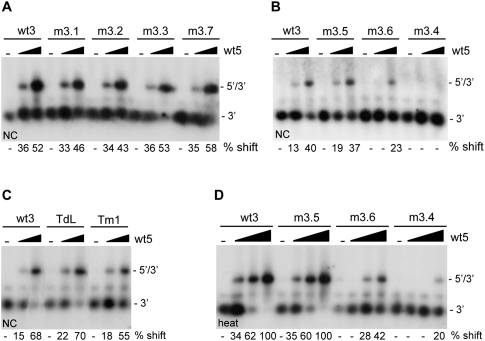
FIGURE 2.
5′–3′ complex formation for the U3R mutants. Equal amounts of the radiolabeled wild-type (wt3) and mutant 3′ transcripts m3.1, 3.2, m3.3, and m3.7 (A), m3.5, m3.6, and m3.4 (B), or TdL and Tm1 (C) were incubated with 0, 50, and 250 nM of the unlabeled 5′ transcript (wt5) in the presence of NC. Subsequently, the NC protein was removed and the samples were analyzed on agarose gels containing TBM. The positions of the radiolabeled 3′ transcript and the 5′–3′ complex are indicated. Quantification by phosphorimaging was used to calculate the percentage of complex formation and indicated below each lane. (D) Equal amounts of the radiolabeled wild-type and mutant 3′ transcripts m3.5, m3.6, and m3.4 were heated together with either 0, 10, 50, or 250 nM of the unlabeled 5′ transcript, followed by slow cooling to room temperature. The samples were analyzed on native TBM gels to measure 3′–5′ complex formation.