Abstract
Although use of methamphetamine (MA) by smoking is the fastest growing method of administration, very limited data are available describing the effects of smoked MA. Using a murine inhalation exposure system, we explored the pulmonary effects of low-dose acute inhalation exposure to MA vapor (smoke). Inhalation of MA vapor resulted in transiently reduced pulmonary function, as measured by transpulmonary resistance, dynamic compliance, and whole-body plethysmography compared with unexposed control animals. These changes were associated with an approximately 34% reduction in serotonin (5-hydroxytryptamine [5-HT]) metabolism/inactivation to 5-hydroxyindolacetic acid, and a nearly 40% reduction in monoamine oxidase (MAO)-A activity in the lung. Pretreatment of mice with a selective 5-HT reuptake inhibitor completely ablated the MA-induced changes in pulmonary function, confirming a key role for the 5-HT transporter (serotonin transporter [SERT]) and the serotonergic system in this effect. Immunofluorescent staining of mouse lung tissue confirmed high expression of SERT in airway epithelial cells. Using mouse airway epithelial cell line, LA-4, and purified human MAO-A, it was demonstrated that MA impedes 5-HT metabolism through direct inhibition of MAO-A activity in vitro. Together, these data demonstrate that low-dose exposure to MA results in reduced pulmonary function mediated via SERT and subsequent perturbation of 5-HT metabolism in the lung. This supports a role for the serotonergic system in MA-mediated pulmonary effects.
Keywords: lung, monoamine oxidase A, selective serotonin reuptake inhibitor, serotonin transporter
CLINICAL RELEVANCE.
The pulmonary effects of exposure to low levels of inhaled methamphetamine (MA) are unknown. Through the use of a murine inhalation model, this study demonstrates that inhalation of low doses of MA vapor (smoke) leads to reduced pulmonary function mediated by the lung serotonergic system. These data support the need for further studies to determine whether a link between MA exposure and respiratory effects exists in humans.
Methamphetamine (MA), a substituted amphetamine with potent central nervous system effects, is currently the most widespread illegally used stimulant in the United States (1). According to the 2007 National Drug Threat Assessment Report by the National Drug Intelligence Center, use by smoking is the fastest growing primary mode of administration (2). Studies of other inhaled drugs, including marijuana and cocaine, have shown that these substances can cause a variety of pulmonary complications. Recently, we demonstrated that an acute inhalation exposure to moderate levels of vaporized MA causes lung injury in mice (3). However, the pulmonary effects of exposure to low levels of inhaled MA are not known. Anecdotal clinical reports have indicated that children removed from homes where MA was smoked have asthma-like symptoms that resolve after removal (R. Shah, personal communication).
Serotonin (5-hydroxytryptamine [5-HT]) has been used for many years as a tool in pulmonary research to induce bronchoconstriction. Although available evidence suggests that most circulating 5-HT is cleared through the lung (4), the biological activity of 5-HT in the lung is not well understood. There is mounting evidence that the serotonergic system may play a role in the pathophysiology of acute asthma. In a study investigating the relationship between plasma levels of free 5-HT with pulmonary function in symptomatic and asymptomatic patients with asthma, free 5-HT levels were closely associated with clinical severity and pulmonary function (5). Furthermore, use of selective serotonin reuptake enhancers (SSREs) provokes a decrease in asthma symptoms and an increase in pulmonary function (6). Unlike selective serotonin uptake inhibitors (SSRIs), which are also used as antidepressants, but instead increase 5-HT levels by blocking reuptake by serotonin reuptake transporter (SERT), SSREs are antidepressants that reduce 5-HT levels by enhancing the uptake of the molecule. These findings have led to speculation that the 5-HT transmitter system might represent novel therapeutic targets for the treatment of asthma.
Under normal physiological conditions, 5-HT levels are tightly regulated through two mechanisms. The 5-HT transporter (SERT) rapidly clears synaptic and circulating 5-HT through transporter-mediated uptake of 5-HT. This transporter is also a target for multiple classes of antidepressants (7, 8) and for drugs of abuse, including cocaine and amphetamine (9). The second mechanism regulating 5-HT levels is intracellular metabolism of 5-HT by monoamine oxidase (MAO). MAO is a key regulatory enzyme for neurotransmitters, drugs, and dietary amines (10). There are two subtypes of this enzyme, which have distinct substrate and inhibitor specificities (11). MAO-A preferentially oxidizes norepinephrine, dopamine, and 5-HT, whereas MAO-B metabolizes phenylethylamine and benzylamine. Both forms of the enzyme oxidize dopamine and tyramine. 5-HT is metabolized via MAO-A through oxidative deamination to 5-hydroxyindole acetic acid (5-HIAA) (12). Ulus and colleagues (13) demonstrated that d-amphetamine and several other related compounds are able to inhibit MAO-A activity in the rat lung. However, to our knowledge, the direct effect of MA administration on MAO-A activity has not been reported.
Recently, Zolkowska and colleagues (14) demonstrated that administration of amphetamine analogs, including MA, resulted in significant increases in plasma 5-HT. The elevated 5-HT, as a result of MA administration, was transient, but high enough to potentially stimulate mitogenic responses in pulmonary artery smooth muscle cells (SMCs), suggesting a possible role for the serotonergic system in MA-mediated pulmonary effects. Based on these findings, we hypothesized that inhalation exposure to low doses of vaporized MA may also result in altered pulmonary function. We further hypothesized that MA-induced lung function changes would likely be mediated via perturbation of the 5-HT system in the lung. The present study was designed to test these hypotheses with our murine exposure model.
MATERIALS AND METHODS
Animals
BALB/c mice used in these studies were bred and maintained in microisolator units in the University of Montana specific pathogen–free animal facility. Mice were allowed food and water ad libitum and were used experimentally at 6–12 weeks of age. All animal use procedures were in accordance with National Institutes of Health and approved by the University of Montana Institutional Animal Care and Use committee.
MA Exposure
Mice were exposed to vapor from 25 mg or 50 mg heated MA with the exposure system described previously (3). Briefly, mice were placed in the chamber and allowed to acclimate for 5 minutes. The heat source was ignited, and the indicated amount of MA (Sigma Chemical Co., St. Louis, MO) heated for 20 minutes. After the 20-minute exposure, the heat source was turned off and the mice remained in the chamber for an additional 5 minutes. Due to risk of low levels of MA exposure, control mice were subjected to the same conditions as the exposed mice (e.g., transfer to and from the exposure chamber), except for being placed inside the exposure chamber. Mice were killed approximately 3 hours after initiation of exposure, except for the timecourse experiment, where pulmonary assessments were conducted between 1 and 24 hours after exposure. For some experiments, mice were pretreated with either saline or the SSRI, citalopram (20 mg/kg intraperitoneal; Sigma), 1 hour before exposure to MA.
Pulmonary Function Assessments
Airway reactivity (AR) to inhaled methacholine was determined by both noninvasive and invasive methods in mice after exposure to MA. Transpulmonary resistance (RL) and dynamic compliance (Cdyn) were assessed as previously described (15). Animals were anesthetized by intraperitoneal injection of ketamine–xylazine, and tracheostomized with insertion of a polyethylene cannula (internal diameter, 0.813 mm). The tracheal tube was connected to a ventilation port within the plethysmograph chamber, and this port was connected to a rodent ventilator (HSE Minivent Type 845; Hugo Sachs Elektronik, Harvard, Germany). Mice were mechanically ventilated at a rate of 120 strokes/min with a stroke volume of 225 μl. Volume changes due to thoracic expansion with ventilation were measured by a transducer connected to the plethysmograph flow chamber. A pressure transducer measured alterations in tracheal pressure as a function of airway caliber. Once stabilized, mice were challenged with saline, followed by increasing concentrations of methacholine (1.5, 3, 6, 12, and 24 mg/ml; Sigma). Aerosols were generated with an ultrasonic nebulizer (Aeroneb Laboratory Nebulizer; Buxco Electronics, Inc., Wilmington, NC) and delivered to the inspiratory line. Each aerosol was delivered for a period of 15 seconds, followed by a 2-minute 45-second period, during which pressure and flow data were continuously recorded. A computer program (BioSystemXA; Buxco Electronics, Inc.) was used to calculate pulmonary RL and Cdyn.
AR was also assessed noninvasively by barometric whole-body plethysmography (WBP; Buxco Electronics, Inc.), as previously described (16). Mice were unrestrained and spontaneously breathing in one of four single-animal chambers while pressure differences between this chamber and a reference chamber were recorded by a barometric analysis technique. In the plethysmograph, mice were allowed to acclimate for 5 minutes, and were then exposed for 3 minutes to nebulized saline and subsequently to increasing concentrations (0, 3, 6, 12, 24, 50 mg/ml) of nebulized methacholine in saline via a DeVilbiss ultrasonic nebulizer (DeVilbiss Healthcare, Somerset, PA). After each nebulization, recordings were taken for 3 minutes, and enhanced pause (Penh) values measured during each 3-minute sequence were averaged. The resulting box pressure signal is caused by volume and pressure changes during the respiratory cycle of the animal, from which the tidal volumes and Penh can be calculated. Penh is a dimensionless value that represents a function of the proportion of maximal expiratory to maximal inspiratory box pressure signals and of the timing of expiration (17). A computer program (BioSystemXA) was used to calculate Penh.
5-HT Metabolism
To determine MA-induced changes in 5-HT metabolism to 5-HIAA in lungs, 5-HT and 5-HIAA levels were measured by reverse-phase HPLC with electrochemical detection. Briefly, lungs from mice exposed to MA were perfused with PBS, snap frozen in liquid nitrogen, and stored at −80°C until being processed. Each lung was homogenized in 1 ml 0.05 M perchloric acid containing 3,4-dihydrobenzylamine (31 ng/ml) as an internal standard. The homogenates were then centrifuged at 14,000 × g for 20 minutes at 4°C, and the supernatants filtered through a Millex hydrophilic LCR (polytetrafluoroethylene) 0.45-μm filter (Millipore, Bedford, MA). Sample filtrates were loaded into vials and placed into an ESA Model 542 autosampler (ESA, Chelmsford, MA). An OmniSpher 5 C18 (Varian, Inc., Lake Forest, CA) chromatographic column with a length of 25 cm and an internal diameter of 4.6 mm was used to separate analytes. The mobile phase consisted of water:acetonitrile (9:1, vol/vol) containing 0.15 M monochloroacetic acid, 0.12 M sodium hydroxide, 0.60 mM EDTA, and 1.30 mM sodium octyl sulfate; the pH was adjusted to 3.2 with glacial acetic acid. A constant flow rate of 1 ml/min was maintained, and the column effluent was analyzed with a Model 5600A ESA CoulArray electrochemical detector (ESA). Potentials of the three ESA Model 6,210 four-channel electrochemical cells, placed in series, were as follows: (channels 1–5) −50, 0, 25, 100, 200 mV; (channels 6–12) 300 mV. 5-HIAA and 5-HT were monitored at 200 mV. Peak area ratios were used to calculate lung 5-HT and 5-HIAA levels from a calibration curve (peak area of analyte/peak area of 3,4-dihydrobenzylamine). 5-HT metabolism is reported as the ratio of 5-HIAA to 5-HT in lung homogenates.
MAO-A Activity
MAO-A activity was assessed in lung tissue homogenates, cell culture homogenates, and purified enzyme preparations with the MAO-Glo assay kit (Promega Corp., Madison, WI), according to the manufacturer's instructions. To determine MAO-A activity in lung tissue, whole lungs were homogenized in 1 ml of cold 0.2 M potassium phosphate buffer (pH 7.6) and used at a 1:4 dilution. For determination of MAO-A activity in mouse lung epithelial cell line, LA-4, cells were plated at 200,000 cells per well in a 6-well plate and allowed to attach overnight. Cells were then cultured for 24 hours with media (control), the MAO-A inhibitor, clorgyline (1 μM), MA (100 μM), or MA plus citalopram (both at 100 μM). After treatment, cells were scraped from the plate in 200 μl cold PBS, sonicated, and used at a 1:2 dilution. Protein levels for each sample were determined with protein assay from Bio-Rad (Hercules, CA), according to the manufacturer's instructions. BSA was used as the standard. MAO-A activity is represented as relative luminescence units per mg protein. To determine the effects of MA on MAO-A activity of a purified enzyme preparation, human MAO-A Supersomes (Gentest, Woburn, MA) at a concentration of 1 mg/ml were used as the MAO-A source. MA (10 μM–100 mM) was incubated with the purified MAO-A for 1 hour, and luminescence was measured. All assays were done in triplicate and reported in relative luminescence units.
Western Blot Analysis
Western blot analysis was performed with mouse brain homogenate supernatant, lung homogenate supernatant, and sonicated LA-4 cell supernatant. Protein level was determined as described previously here. Protein extract (10 μg) was separated on a NuPAGE 4–12% Bis-Tris gel (Invitrogen Life Technologies, Carlsbad, CA) and electrophoretically transferred to Immun-Blot PVDF membrane (Bio-Rad). The membrane was blocked with 5% nonfat dry milk in Tris-buffered saline containing 0.1% Tween. After blocking, the membrane was incubated overnight at 4°C with a 1:500 dilution of rabbit anti-SERT (Abcam Inc., Cambridge, MA). For blocking studies, the membrane was incubated overnight at 4°C with a 1:1,000 dilution of goat anti-SERT (Santa Cruz Biotechnology, Santa Cruz, CA), or goat anti-SERT preincubated for 1 hour on ice with the blocking peptide (Santa Cruz Biotechnology) was used. The membrane was washed with Tris-buffered saline containing 0.1% Tween, and then incubated for 1 hour at room temperature with a 1:1,000 dilution of goat anti-rabbit IgG–horseradish peroxidase conjugate (Pierce Laboratories, Rockford, IL) or a 1:25,000 dilution of rabbit anti-goat IgG–horseradish peroxidase conjugate (Thermo Fisher Scientific, Waltham, MA). Bands were visualized by chemiluminescence (SuperSignal West Femto Substrate Kit; Pierce Laboratories) on a Versa Doc Imaging System (Bio-Rad). Band intensity was quantified with Bio-Rad Quantity One software. A protein standard ladder (MagicMark XP Western Standards; Invitrogen Life Technologies) was used for estimation of protein molecular size.
Histopathology
The lungs and trachea were exposed by thoracotomy. Lungs were inflation fixed through the trachea with 3% paraformaldehyde–PBS and then submerged in 3% paraformaldehyde–PBS overnight at 4°C. The lungs were washed with cold PBS, processed, embedded in paraffin blocks, serially sectioned at 7 μm, and mounted in a 1-in-10 series on Superfrost Plus slides (VWR, West Chester, PA). Lung sections were deparaffinized, fixed in ice-cold acetone, permeabilized with Triton-X, blocked with BSA in PBS, and stained with a 1:1,000 dilution of rabbit anti-SERT (Abcam Inc.) overnight at 4°C. Immunoreactivity was revealed by staining with goat anti-rabbit secondary antibody (Alexa Fluor 633; Invitrogen Life Technologies). As a negative control, some sections were stained only with goat anti-rabbit secondary antibody.
Statistical Analyses
The mean (±SEM) was calculated for all samples, and P values calculated using a one-way ANOVA, followed by Dunnett's multiple comparison to a single control group. For the pulmonary function assessments, statistical analysis by two-factor ANOVA was conducted to determine effects of MA exposure and methacholine doses on pulmonary function measurements.
RESULTS
Pulmonary Function after Exposure to MA Vapor
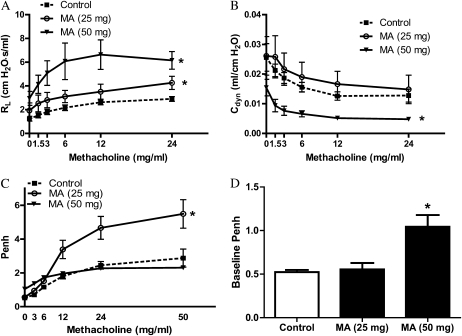
To determine whether acute exposure to MA vapor alters pulmonary function in mice, AR was assessed by both invasive and noninvasive methods. RL and Cdyn values in intubated, anesthetized mice are shown in Figures 1A and 1B, respectively. Determination of RL and Cdyn by this method provides reproducible information regarding AR and pulmonary mechanics in mice (18). Methacholine challenge resulted in dose-dependent increases in RL and decreases in Cdyn for both the control and MA-exposed groups. Analysis by a two-factor ANOVA demonstrated significantly increased RL after 25- and 50-mg MA exposures compared with control mice, and significantly decreased Cdyn after the 50-mg MA exposure compared with control animals. In addition, there was a significant difference in the baseline RL between control mice and the mice exposed to 50 mg MA (1.24 ± 0.21 versus 2.95 ± 0.58; P < 0.05). Although changes in baseline RL can have an effect on airway responsiveness, these data support the notion that, even with increased baseline RL, the mice exposed to 50 mg MA exhibited enhanced airway hyperresponsiveness compared with control mice.
Figure 1.
Pulmonary function 3 hours after exposure to methamphetamine (MA) vapor. Pulmonary function was assessed as a function of increasing methacholine dose in live mice 3 hours after exposure to MA. (A and B) Transpulmonary resistance (RL [A]) and dynamic compliance (Cdyn [B]) were assessed after anesthetization and tracheostomy (n = 10 for control; n = 5 for 25 mg MA; and n = 6 for 50 mg MA). Statistical analysis by two-factor ANOVA shows significant effects for 25- and 50-mg concentrations (RL), and 50 mg MA (Cdyn) compared with control animals. *P < 0.05. (C and D) Barometric whole-body plethysmography (WBP) was assessed in spontaneously breathing mice (n = 4 [C]). For the 25-mg dose, statistical analysis by two-factor ANOVA shows significant effects for MA treatment compared with control animals (*P < 0.05). Baseline enhanced pause (Penh) is shown in D (n = 4). Baseline Penh is expressed as means (±SE). *P < 0.05 versus control animals.
To confirm these findings in spontaneously breathing mice, WBP was also conducted after MA exposure. This method allows repeated assessments of airway hyperreactivity. Exposure to 25 mg MA resulted in significantly increased AR to methacholine compared with control animals (Figure 1C). Responsiveness to methacholine was not observed after exposure to 50 mg MA. However, similar to the changes observed in baseline lung resistance, the baseline Penh value was nearly twofold higher than in control animals after this higher dose, confirming altered baseline AR in the animals exposed to 50 mg MA.
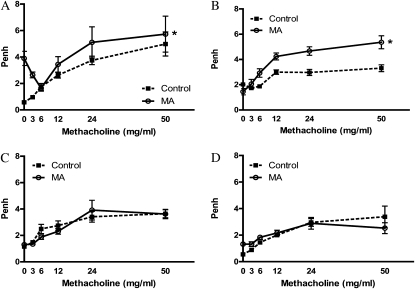
WBP was measured at various time points in the same mice after exposure to determine whether elevated AR in mice exposed to MA was persistent or transient. WBP was assessed at 1 hour (Figure 2A), 3 hours (Figure 2B), 5 hours (Figure 2C), and 24 hours (Figure 2D) in control mice and in mice exposed to 25 mg MA. Analysis by a two-factor ANOVA demonstrated significant effects of MA exposure at the two earliest time points. By 5 hours after exposure, AR returned to that observed in the control mice. Although it is possible that repeated methacholine challenges in the same mice may blunt responses over time, no significant changes in methacoline responses were noted in the control mice over time, suggesting that repeated challenges have little effect on AR. These data confirm that the pulmonary effects seen in mice acutely exposed to MA are transient in nature.
Figure 2.
Timecourse of airway hyperresponsiveness after exposure to MA vapor. WBP was assessed 1 (A), 3 (B), 5 (C), and 24 (D) hours after inhalation exposure to MA. To determine changes in Penh over time, the same animals were used for each time point (25 mg; n = 4). Statistical analysis by two-factor ANOVA shows significant effects for MA treatment at 1 and 3 hours compared with control animals (*P < 0.05).
5-HT Metabolism in the Lung after Exposure to MA Vapor
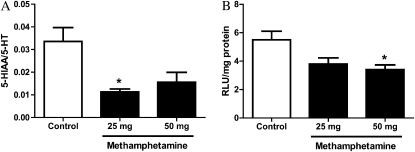
Zolkowska and colleagues (14) reported that intravenous administration of MA in rats resulted in increased plasma 5-HT concentrations. Given 5-HT is an effective bronchoconstrictor and other amphetamine analogs have been shown to inhibit 5-HT metabolism to 5-HIAA (13), we assessed whether 5-HT metabolism was altered in lungs from MA-exposed mice. As shown in Figure 3A, 5-HT metabolism was significantly decreased 3 hours after exposure to 25 mg MA. Exposure to 50 mg MA also appeared to decrease 5-HT metabolism; however, this was not statistically significant. To determine whether reduced MAO-A activity could contribute to decreased 5-HT metabolism, MAO-A activity was determined in lung homogenates from exposed and control mice (Figure 3B). After exposure to 50 mg MA, MAO-A activity was significantly decreased by 38% compared with control animals. Exposure to 25 mg MA also reduced lung MAO-A activity; however, this reduction did not reach statistical significance.
Figure 3.
Serotonin (5-hydroxytryptamine [5-HT]) metabolism in the lung after exposure to MA vapor. At 3 hours after exposure to MA, lungs were collected and assessed for 5-HT metabolism (5-hydroxyindole acetic acid [5-HIAA]/5-HT [A]) and monoamine oxidase (MAO)-A activity (B). (A) 5-HT and 5-HIAA levels in perfused lung tissue homogenates were measured by reverse-phase HPLC with electrochemical detection (control, n = 10; 25 mg, n = 5; 50 mg, n = 6). (B) MAO-A activity in whole-lung homogenates was assessed by luminescence, as described in the Materials and Methods (control, n = 14; MA-treated, n = 7). Data presented are means (±SE); *P < 0.05 versus control animals. RLU, relative luminescence units.
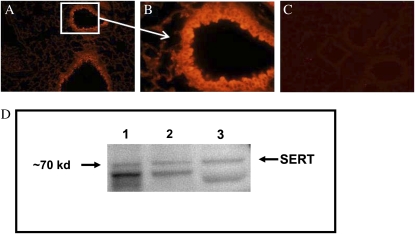
Figure 5.
Serotonin transporter (SERT) expression in mouse lung epithelial cells. Immunofluorescent staining of lung sections from normal Balb/c mice revealed significant expression of SERT in airway epithelial cells (A and B). Controls with secondary antibody only show low background staining (C). A and C, 200×; B, 600×. Immunoblotting was used to determine whether SERT is expressed in the mouse lung epithelial cell line, LA-4 (D). Lane 1 contains mouse brain homogenate; lane 2 contains mouse lung homogenate; and lane 3 contains lysate from LA-4 cells. A strong band was detected at approximately 68 kD, and another, fainter band at approximately 70 kD. The predicted molecular weight of SERT is 70.3 kD.
Effect of Citalopram Pretreatment on Airway Changes in Mice
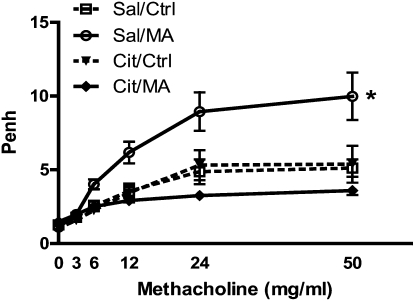
To confirm that the serotonergic system plays a role in the airway effects observed after MA exposure, the effect of pretreating the mice with an SSRI to block transport of 5-HT was determined. Mice were given intraperitoneal injections of either saline or citalopram 1 hour before exposure to MA. WBP was assessed after methacholine challenge (Figure 4). Pretreatment with citalopram completely ablated the MA-induced changes in AR. Pretreatment of control mice with citalopram appeared to slightly diminish the responsiveness to methacholine. The ability of citalopram to block the effects of MA on AR was confirmed by lung resistance measurements (data not shown). These results support a key role for the lung serotonergic system in the pulmonary effects after MA exposure.
Figure 4.
Effect of citalopram pretreatment on airway changes in mice. WBP was assessed 3 hours after inhalation exposure to MA (25 mg). Animals were pretreated with either saline (Sal) or citalopram (Cit; 20 mg/kg, intraperitoneal) 1 hour before exposure (Sal, n = 12; Cit, n = 8). Statistical analysis by two-factor ANOVA shows significant treatment effect for Sal/MA compared with Sal/Ctrl (*P < 0.05). Ctrl, control.
SERT Expression in Mouse Lung Epithelial Cells
Inhibition of the MA-induced AR in the lung with citalopram suggested a key role for SERT in the observed pulmonary effects. It is known that SERT is highly expressed in the lung. In particular, its expression has been documented in pulmonary artery endothelial cells and SMCs (19, 20). To determine the localization of SERT in mouse lung tissues, lung sections were stained with an antibody specific to the mouse SERT (Figures 5A–5B). Intense staining was observed in the lung epithelial cells lining both large and small airways. Some staining in airway SMCs also appeared visible, although this was difficult to distinguish from the intense epithelial cell signal. Less intense SERT staining was also observed in the endothelial cell layer of vessels (data not shown). Expression of SERT protein in epithelial cells was confirmed by Western blot in a mouse lung epithelial cell line (LA-4; Figure 5D). Expression of SERT protein in LA-4 cells was similar to that seen in mouse brain and lung homogenates. These results suggest that lung epithelial cells may play an important role in uptake of 5-HT in the lung. A specific blocking peptide was used to determine specificity of the SERT antibody in the Western blot. The blocking peptide significantly blocked SERT binding in mouse brain homogenate and LA-4 cell lysates on Western blots (see Figure E1 in the online supplement).
Effect of MA on MAO-A Activity In Vitro
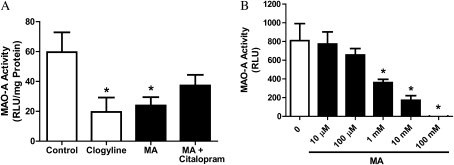
The aforementioned results demonstrate that there is significant MAO-A activity in whole-lung homogenates, and lung epithelial cells express SERT protein. We next explored whether epithelial cells themselves may have significant MAO-A activity, and whether MA could affect MAO-A activity in epithelial cells in vitro. LA-4 cells were cultured for 24 hours in the presence of MA with or without citalopram (Figure 6A). The specific MAO-A inhibitor, clorgyline, was used as a positive control for MAO-A inhibition. As was observed in the lung in vivo, exposure to MA resulted in a significant decrease in MAO-A activity. This inhibition was partially reversed by the addition of citalopram, indicating that the effects of MA are likely through (at least in part) SERT. To determine whether MA directly inhibits the MAO-A enzyme, or if this inhibition was indirect through some other control mechanism, human recombinant MAO-A microsomes were used (Figure 6B). The addition of MA dose-dependently inhibited the activity of purified MAO-A, confirming that the effect of MA on the MAO-A enzyme is direct.
Figure 6.
Effect of MA on MAO-A activity in vitro. (A) LA-4 cells were cultured for 24 hours with the MAO-A inhibitor, clorgyline (1 μM), or MA (100 μM), with or without the selective serotonin uptake inhibitor (SSRI), citalopram (100 μM), and MAO-A activity of cell lysates was assessed (n = 6). Data presented are means (±SE); *P < 0.05 versus control cells. (B) The ability of MA to directly inhibit MAO-A activity was assessed with purified human MAO-A enzyme. Purified MAO-A was incubated in the presence of increasing levels of MA and MAO-A activity assayed by a luminescence method, as described in Materials and Methods. MA at concentrations of 1 mM and higher significantly reduced the activity of purified human MAO-A (n = 3). Data presented are means (±SE); *P < 0.05 versus control animals.
DISCUSSION
We previously reported that acute inhalation exposure to MA vapor results in dose-dependent lung injury in mice (3). Although it is difficult to draw comparisons between exposures in humans and mice, in our previous work exploring lung injury after exposure to 25 to 100 mg MA, the doses of MA causing lung injury resulted in plasma MA levels that were well below the mean MA plasma level of 730 ng/ml reported for MA users (21). However, it is likely that the findings in our initial study more closely modeled the respiratory effects expected to be seen in MA users rather than in environmental exposures. In the present study, we explored the respiratory effects of doses resulting in the lowest detectable MA plasma levels as a more close approximation of environmental exposures. To this end, we used the same exposure system to determine whether lower doses of inhaled MA could affect pulmonary function. Results from this study demonstrate that inhalation exposure to low doses of MA (25–50 mg, which results in MA plasma concentrations of approximately 6.75 and 35.30 ng/ml, respectively [3]) leads to transiently altered pulmonary function in mice.
Children removed from environments where MA has been manufactured or used often present with transient asthma-like symptoms, including airway hyperresponsiveness (R. Shah, personal communication). Data from this study support the notion that exposure to MA may contribute to these observed effects. Although the pathophysiology of asthma is complex, data are accumulating that support a role for 5-HT in the pathophysiology of this disease. In patients with asthma, free 5-HT levels have been positively correlated with bronchoconstriction and clinical severity (5). It was subsequently shown that tianeptine, an SSRE that reduces free 5-HT through enhancing uptake, provokes a rapid and significant decrease of both clinical rating and free 5-HT plasma levels associated with improved pulmonary function (6, 22). Conversely, buspirone, which increases free 5-HT in plasma, triggered asthma attacks in patients with asthma (23). The recent finding that intravenous administration of MA to rats results in a transient, significant increase in plasma 5-HT prompted us to explore whether the increased AR observed in mice after inhalation exposure to low-dose MA could be related to perturbation of the serotonergic system in the lung. Indeed, pretreating mice with the SSRI, citalopram, completely blocked the MA-mediated airway hyperresponsiveness, providing strong evidence for a key role for the serotonergic system in this effect.
It has been recently demonstrated that several antidepressants, including citalopram, inhibit SERT via a competitive mechanism with 5-HT (24). Evidence suggests that MA, which also acts as a substrate for SERT, both blocks transmitter uptake as well as stimulates 5-HT release (25, 26). We hypothesize that pretreatment of the mice with citalopram blocks the uptake of MA and subsequent release of 5-HT. The finding that citalopram in vitro was able to partially reverse the effects of MA on MAO-A activity further supports the concept that MA enters epithelial cells via SERT, and this is blocked by the reuptake inhibitor, citalopram. As demonstrated by Zolkowska and colleagues (14), SSRIs may themselves cause transient increases in circulating 5-HT. However, these transient increases resolve within 60 minutes after administration. In the present study, mice were pretreated with citalopram 1 hour before exposure to MA. Therefore, we would expect that any potential citalopram-induced transient increases in circulating 5-HT would have resolved by this time.
Free 5-HT is rapidly taken up and cleared by the lungs through the action of SERT. In a study of exogenously infused 5-HT, it was shown that lungs are able to clear 70–90% of the 5-HT during one passage through the pulmonary circulation (27). In the lung, SERT is expressed at high levels (28), and is thought to be predominantly found in pulmonary artery endothelial cells and SMCs (19, 20). Based on the histological findings in this study, in addition to endothelial cells and SMCs, mouse lung epithelial cells also have significant SERT expression. This finding was further supported by the presence of SERT protein in the mouse lung epithelial cell line, LA-4. Based on these data, enhanced AR observed after MA exposure in our model may involve SERT-mediated exchange of drug molecules for epithelial 5-HT. Within the pulmonary system, potential sources of 5-HT include platelets, mast cells, and pulmonary neuroendrocrine cells. The data presented here suggest that lung epithelial cells may also be a significant source of 5-HT in the lung. Further studies will be necessary to confirm whether lung epithelial cells can contribute to overall lung 5-HT content, or whether their role is primarily one of uptake and metabolism. Taken together, our data demonstrate an important role for epithelial cells in the maintenance of lung 5-HT levels.
The present finding, that inhalation of MA leads to MAO-A inhibition in the lung and results in reduced 5-HT metabolism, suggests a second mechanism for the observed effect of MA on pulmonary function. Once the cell has been exposed to MA, MAO-A activity may be directly inhibited, leading to increased intracellular 5-HT potentially available for release. Using purified human MAO-A, we further demonstrated that the effect of MA on MAO-A activity is direct. To our knowledge, this is the first demonstration of the direct inhibition of MAO-A activity by MA, although it has been previously shown that d-amphetamine and several other related compounds are capable of inhibiting MAO-A activity both directly and in the rat lung (13). Furthermore, studies indicating that cigarette smokers have reduced MAO-A levels in the brain (29) and lung (30) suggest a more generalized role for the inhibition of MAO-A activity in physiological effects in the lung.
In this study, we used both noninvasive and invasive methods for assessing the pulmonary function in mice exposed to MA. Invasive monitoring of lung function by RL and Cdyn is the classical method for accurate and specific determination of pulmonary mechanics (31). However, this method does not enable the repeated measure of pulmonary function. Therefore, noninvasive assessment by WPB (reported as Penh) was also employed to measure AR, so that persistence of pulmonary function changes could be monitored over time. Through the use of both methods, our results confirm that inhalation exposure to MA significantly altered pulmonary function. However, by these two methods, we observed different patterns in altered lung function after exposure. Elevated pulmonary resistance to increasing methacholine doses, as measured by RL, was observed after exposure to both 25 and 50 mg MA. With WBP, however, AR, after exposure to 50 mg MA, resulted in altered baseline lung function and a loss of responsiveness to methacholine. This could be related to the possibility that measurements of Penh originate as part of reflex control of breathing processes, rather than completely in the lung mechanics (32). Nonetheless, results from both methods confirm that inhalation exposure to MA results in significantly altered pulmonary function.
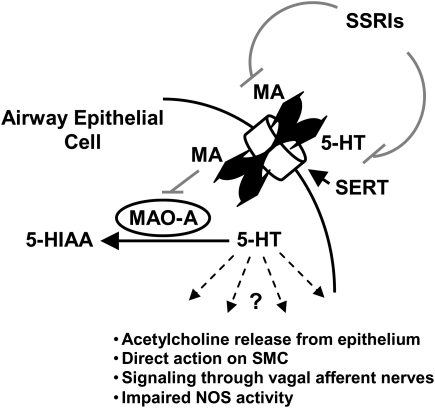
The data described here provide direct evidence that the serotonergic system plays an important role in lung function and pathophysiology after exposure to inhaled MA. More specifically, low-dose exposure to MA results in significantly reduced pulmonary function that can be ablated by inhibition of SERT, and is related to changes in 5-HT metabolism in the lung. A model describing the proposed mechanism of this effect is shown in Figure 7. In this model, MA enters lung epithelial cells through SERT. This results in a transient elevation of 5-HT through both the direct inhibition of MAO-A activity by MA and the immediate SERT-mediated efflux of cytoplasmic 5-HT. Pretreatment with the SSRI, citalopram, ablates these effects by blocking the SERT-mediated transport of MA into the cell and 5-HT extrusion. MA-mediated changes in 5-HT metabolism/efflux then affects bronchoconstriction via downstream mechanisms, which may include acetylcholine release from the epithelium (33), direct action of 5-HT on SMC (34), 5-HT signaling through vagal afferent nerves (35, 36), and/or impaired nitric oxide release as a result of the direct interaction between SERT and nitric oxide synthase (37).
Figure 7.
Proposed model for mechanism of MA-mediated perturbation of 5-HT metabolism in the lung. In this model, MA enters lung epithelial cells through SERT. This results in a transient elevation of 5-HT through both the direct inhibition of MAO-A activity by MA and the immediate SERT-mediated efflux of cytoplasmic 5-HT. Pretreatment with the SSRI, citalopram, ablates these effects by blocking the SERT-mediated transport of MA into the cell and 5-HT extrusion. MA-mediated changes in 5-HT metabolism/efflux then affects bronchoconstriction via downstream mechanisms, which may include acetylcholine release from the epithelium (33), direct action of 5-HT on smooth muscle cell (SMC) (34), 5-HT signaling through vagal afferent nerves (35, 36), and/or impaired nitric oxide (NO) release as a result of the direct interaction between SERT and NO synthase (NOS) (37).
The findings presented here support the need to conduct further studies to determine whether there may be a link between MA exposures and respiratory effects in humans. In addition, the exposures in the study were acute. Given that most direct and indirect exposure to MA are likely chronic in nature, additional studies of the effects of chronic, repeated exposure to MA are warranted. Demonstrating that inhalation of low doses of MA can result in a 5-HT–mediated reduction in pulmonary function should prove useful for consideration of improved respiratory treatment of patients removed from situations where MA exposures may have occurred.
Supplementary Material
Acknowledgments
The authors thank R. Hamilton for statistical support, B. Postma for technical assistance with the laboratory animals, L. Herritt for technical assistance with histology and microscopy, and E. Klein for technical assistance with the biochemical assays.
This work was supported by National Institutes of Health (NIH)/National Center for Research Resources (NCRR) grants P20-RR-017670 and P20-RR-015583, National Heart, Lung, and Blood Institute grants K99-HL-088550 and R00-HL-088550 (S.M.W.), and Centers for Disease Control and Prevention/National Center for Environmental Health grant 1H75-EH-000378 (A.H.).
The contents of this work are solely the responsibility of the authors and do not necessarily represent the official views of the National Center for Research Resources or the National Institutes of Health.
This article has an online supplement, which is accessible from this issue's table of contents at www.atsjournals.org
Originally Published in Press as DOI: 10.1165/rcmb.2009-0121OC on June 18, 2009
Conflict of Interest Statement: F.C.-P. received a sponsored grant from National Institute on Aging ($100,001 or more). A.H. received a sponsored grant from COBRE for cores that generated data from the National Institutes of Health (NIH) ($100,001 or more) and a direct support grant to conduct research from Centers for Disease Control ($100,001 or more). S.M.W. received a sponsored grant from the NIH ($100,001 or more). None of the other authors has a financial relationship with a commercial entity that has an interest in the subject of this manuscript.
References
- 1.National Institute on Drug Abuse. Methamphetamine abuse and addiction. Bethesda, MD: National Institutes of Health; 2006.
- 2.National Drug Intelligence Center. National drug threat assessment 2007. Washington, DC: US Department of Justice; 2007.
- 3.Wells SM, Buford MC, Braseth SN, Hutchison JD, Holian A. Acute inhalation exposure to vaporized methamphetamine causes lung injury in mice. Inhal Toxicol 2008;20:829–838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wiersma DA, Roth RA. Clearance of 5-hydroxytryptamine by rat lung and liver: the importance of relative perfusion and intrinsic clearance. J Pharmacol Exp Ther 1980;212:97–102. [PubMed] [Google Scholar]
- 5.Lechin F, van der Dijs B, Orozco B, Lechin M, Lechin AE. Increased levels of free serotonin in plasma of symptomatic asthmatic patients. Ann Allergy Asthma Immunol 1996;77:245–253. [DOI] [PubMed] [Google Scholar]
- 6.Lechin F, van der Dijs B, Orozco B, Jara H, Rada I, Lechin ME, Lechin AE. The serotonin uptake–enhancing drug tianeptine suppresses asthmatic symptoms in children: a double-blind, crossover, placebo-controlled study. J Clin Pharmacol 1998;38:918–925. [DOI] [PubMed] [Google Scholar]
- 7.Owens MJ, Morgan WN, Plott SJ, Nemeroff CB. Neurotransmitter receptor and transporter binding profile of antidepressants and their metabolites. J Pharmacol Exp Ther 1997;283:1305–1322. [PubMed] [Google Scholar]
- 8.Tatsumi M, Groshan K, Blakely RD, Richelson E. Pharmacological profile of antidepressants and related compounds at human monoamine transporters. Eur J Pharmacol 1997;340:249–258. [DOI] [PubMed] [Google Scholar]
- 9.Amara SG, Sonders MS. Neurotransmitter transporters as molecular targets for addictive drugs. Drug Alcohol Depend 1998;51:87–96. [DOI] [PubMed] [Google Scholar]
- 10.Strolin Benedetti M, Tipton KF. Monoamine oxidases and related amine oxidases as phase I enzymes in the metabolism of xenobiotics. J Neural Transm Suppl 1998;52:149–171. [DOI] [PubMed] [Google Scholar]
- 11.Shih JC, Chen K, Ridd MJ. Monoamine oxidase: from genes to behavior. Annu Rev Neurosci 1999;22:197–217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Molinoff PB, Axelrod J. Biochemistry of catecholamines. Annu Rev Biochem 1971;40:465–500. [DOI] [PubMed] [Google Scholar]
- 13.Ulus IH, Maher TJ, Wurtman RJ. Characterization of phentermine and related compounds as monoamine oxidase (MAO) inhibitors. Biochem Pharmacol 2000;59:1611–1621. [DOI] [PubMed] [Google Scholar]
- 14.Zolkowska D, Rothman RB, Baumann MH. Amphetamine analogs increase plasma serotonin: implications for cardiac and pulmonary disease. J Pharmacol Exp Ther 2006;318:604–610. [DOI] [PubMed] [Google Scholar]
- 15.Ameredes BT. Cardiac activity during airway resistance alterations with intravenous and inhaled methacholine. Respir Physiol Neurobiol 2004;139:281–292. [DOI] [PubMed] [Google Scholar]
- 16.Archer AJ, Cramton JL, Pfau JC, Colasurdo G, Holian A. Airway responsiveness after acute exposure to urban particulate matter 1648 in a DO11.10 murine model. Am J Physiol Lung Cell Mol Physiol 2004;286:L337–L343. [DOI] [PubMed] [Google Scholar]
- 17.Chong BT, Agrawal DK, Romero FA, Townley RG. Measurement of bronchoconstriction using whole-body plethysmograph: comparison of freely moving versus restrained guinea pigs. J Pharmacol Toxicol Methods 1998;39:163–168. [DOI] [PubMed] [Google Scholar]
- 18.Glaab T, Ziegert M, Baelder R, Korolewitz R, Braun A, Hohlfeld JM, Mitzner W, Krug N, Hoymann HG. Invasive versus noninvasive measurement of allergic and cholinergic airway responsiveness in mice. Respir Res 2005;6:139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Eddahibi S, Humbert M, Fadel E, Raffestin B, Darmon M, Capron F, Simonneau G, Dartevelle P, Hamon M, Adnot S. Serotonin transporter overexpression is responsible for pulmonary artery smooth muscle hyperplasia in primary pulmonary hypertension. J Clin Invest 2001;108:1141–1150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lee SL, Fanburg BL. Serotonin uptake by bovine pulmonary artery endothelial cells in culture. II. Stimulation by hypoxia. Am J Physiol 1986;250:C766–C770. [DOI] [PubMed] [Google Scholar]
- 21.Schwilke EW, Sampaio dos Santos MI, Logan BK. Changing patterns of drug and alcohol use in fatally injured drivers in Washington state. J Forensic Sci 2006;51:1191–1198. [DOI] [PubMed] [Google Scholar]
- 22.Lechin F, van der Dijs B, Orozco B, Jara H, Rada I, Lechin ME, Lechin AE. Neuropharmacologic treatment of bronchial asthma with the antidepressant tianeptine: a double-blind, crossover placebo-controlled study. Clin Pharmacol Ther 1998;64:223–232. [DOI] [PubMed] [Google Scholar]
- 23.Lechin F, van der Dijs B, Jara H, Orozco B, Baez S, Benaim M, Lechin M, Lechin A. Effects of buspirone on plasma neurotransmitters in healthy subjects. J Neural Transm 1998;105:561–573. [DOI] [PubMed] [Google Scholar]
- 24.Apparsundaram S, Stockdale DJ, Henningsen RA, Milla ME, Martin RS. Antidepressants targeting the serotonin reuptake transporter (SERT) act via a competitive mechanism. J Pharmacol Exp Ther 2008;327:982–990. [DOI] [PubMed] [Google Scholar]
- 25.Rothman RB, Baumann MH. Monoamine transporters and psychostimulant drugs. Eur J Pharmacol 2003;479:23–40. [DOI] [PubMed] [Google Scholar]
- 26.Rudnick G, Clark J. From synapse to vesicle: the reuptake and storage of biogenic amine neurotransmitters. Biochim Biophys Acta 1993;1144:249–263. [DOI] [PubMed] [Google Scholar]
- 27.Davis RB, Wang Y. Rapid pulmonary removal of 5-hydroxytryptamine in the intact dog. Proc Soc Exp Biol Med 1965;118:797–800. [DOI] [PubMed] [Google Scholar]
- 28.Fowler JS, Logan J, Wang GJ, Franceschi D, Volkow ND, Telang F, Pappas N, Ferrieri R, Shea C, Garza V, et al. Monoamine oxidase A imaging in peripheral organs in healthy human subjects. Synapse 2003;49:178–187. [DOI] [PubMed] [Google Scholar]
- 29.Fowler JS, Volkow ND, Wang GJ, Pappas N, Logan J, Shea C, Alexoff D, MacGregor RR, Schlyer DJ, Zezulkova I, et al. Brain monoamine oxidase A inhibition in cigarette smokers. Proc Natl Acad Sci USA 1996;93:14065–14069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fowler JS, Logan J, Wang GJ, Volkow ND, Telang F, Zhu W, Franceschi D, Shea C, Garza V, Xu Y, et al. Comparison of monoamine oxidase A in peripheral organs in nonsmokers and smokers. J Nucl Med 2005;46:1414–1420. [PubMed] [Google Scholar]
- 31.Glaab T, Taube C, Braun A, Mitzner W. Invasive and noninvasive methods for studying pulmonary function in mice. Respir Res 2007;8:63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Adler A, Cieslewicz G, Irvin CG. Unrestrained plethysmography is an unreliable measure of airway responsiveness in BALB/c and C57BL/6 mice. J Appl Physiol 2004;97:286–292. [DOI] [PubMed] [Google Scholar]
- 33.Cazzola I, Matera MG. 5-HT modifiers as a potential treatment of asthma. Trends Pharmacol Sci 2000;21:13–16. [DOI] [PubMed] [Google Scholar]
- 34.Kummer W, Wiegand S, Akinci S, Wessler I, Schinkel AH, Wess J, Koepsell H, Haberberger RV, Lips KS. Role of acetylcholine and polyspecific cation transporters in serotonin-induced bronchoconstriction in the mouse. Respir Res 2006;7:65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bhattacharya BK. A pharmacological study on the effect of 5-hydroxytryptamine and its antagonists on the bronchial musculature. Arch Int Pharmacodyn Ther 1955;103:357–369. [PubMed] [Google Scholar]
- 36.Islam MS, Melville GN, Ulmer WT. Role of atropine in antagonizing the effect of 5-hydroxytryptamine (5-HT) on bronchial and pulmonary vascular systems. Respiration 1974;31:47–59. [DOI] [PubMed] [Google Scholar]
- 37.Chanrion B, Mannoury la Cour C, Bertaso F, Lerner-Natoli M, Freissmuth M, Millan MJ, Bockaert J, Marin P. Physical interaction between the serotonin transporter and neuronal nitric oxide synthase underlies reciprocal modulation of their activity. Proc Natl Acad Sci USA 2007;104:8119–8124. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.