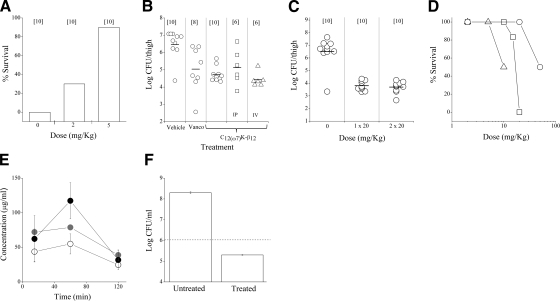
Figure 6.
In vivo studies in mice. A) Efficacy of C12(ω7)K-β12 in protecting mice from a lethal challenge using the peritonitis-sepsis model. ICR mice were inoculated IP with 1 × 108 CFU of S. aureus (CI 15903) and treated by i.p. injection 1 h postinoculation. Number of mice per group is specified in brackets. Data are values at 6 d postinoculation. B, C) Ability of C12(ω7)K-β12 to affect bacterial viability systemically using the thigh infection model. Mice were inoculated intramuscularly with 1.4–1.5 × 106 CFU of S. aureus (ATCC 29213) and treated 2 h after (B) or immediately after inoculation (C). Vanco, vancomycin. Unless specified otherwise, treatments were s.c. Horizontal bars indicate mean values. D) Acute toxicity of C12(ω7)K-β12 at 6 d after various routes of administrations. n = 2 mice/group for highest doses; 6 mice/group for all lower doses. Triangles, i.v. route; rectangles, i.p. route; circles, s.c. route. E) Blood concentration of C12(ω7)K-β12 as determined after extraction and analysis by LC-MS for single-dose administrations of 5, 10, or 20 mg/kg (open, shaded, and solid circles, respectively). Data are means ± sd from 2 mice/data point. F) Inhibition of S. aureus (CI 15903) after 12 h incubation with C12(ω7)K-β12 (20 μg/ml) in 90% human whole blood. Horizontal dashed line indicates initial inoculum.