Abstract
Hepatocyte growth factor (HGF) and its receptor MET have been implicated in uterine development, pregnancy, and endometrial disorders, such as endometriosis and carcinoma. In vitro studies have shown that HGF acts as a mitogen, motogen, and morphogen on endometrial epithelial cells. However, the expression and regulation of HGF and MET in the uteri of different species remain obscure. The present study aimed to investigate the changes of HGF, MET, and HGF activator (HGFA) expression in the uterine endometrium during the estrous cycle in mice and to explore estrogen and progesterone regulation of their expression. MKI67 immunostaining was conducted to examine the association between HGF/MET expression and endometrial cell proliferation. Endometrial epithelial and stromal cells both expressed HGF, HGFA, and MET, but the cell type-specific patterns changed during the cycle. Estrogen and progesterone differentially regulated HGF, MET, and HGFA expression. Progesterone up-regulated their expression in the stroma and down-regulated their expression in the luminal epithelium, whereas 17-beta-estradiol down-regulated their expression in the glandular epithelium. The pattern of HGF/MET overall correlated with that of MKI67. In conclusion, HGF, HGFA, and MET expression in mouse uterus changes during the estrous cycle in a stage-, cell type-, and compartment-specific manner under the influence of estrogen and progesterone. HGF likely plays a role in cyclic endometrial remodeling, such as cell proliferation via autocrine/paracrine mechanisms in mouse uterus.
Keywords: estradiol, female reproductive tract, growth factors, progesterone, uterus
The hepatocyte growth factor system is regulated in cycling mouse uterus by estrogen and progesterone in a stage-, cell type-, and compartment-specific manner.
INTRODUCTION
Hepatocyte growth factor (HGF) is a multifunctional growth factor that displays remarkable mitogenic, motogenic, morphogenic, and antiapoptotic activities in a variety of cells, mainly through its receptor MET tyrosine kinase. Data from Hgf transgenic mice indicate that overexpression of HGF leads to abnormal development and tumor formation [1]. Previous studies have implicated HGF in multiple processes, including trophoblast cell proliferation during implantation [2], uterine epithelial cell morphogenesis during pregnancy [3], postnatal development [4] and in vitro cell culture [5], and progression of endometrial cancer [6] and endometriosis [7].
Despite the importance of HGF and MET in uterine function, the regulation of their expression in the uteri of different species remains obscure. The dogma is that HGF is a stromal-derived growth factor in the endometrium, where it functions in a paracrine way through MET in the epithelial cells. However, HGF protein has been localized to both normal human endometrial epithelium and endometrial carcinoma cells [8]. In cycling or pregnant ovine uterus, HGF mRNA is expressed in the endometrial stroma, with higher levels in the stratum compactum of the intercaruncular endometrium, whereas MET mRNA is expressed exclusively in the luminal and glandular epithelium (GE) [9]. The variations of their abundance in ovine uterus during the estrous cycle and pregnancy suggest complex actions of ovarian steroids and/or cytokines. A more recent study revealed an up-regulation of MET mRNA in the GE in ovine endometrium by progesterone (P4) [10]. Lindsey and Brenner [11] reported that HGF mRNA levels are regulated by estrogen and P4 in primate endometrium, but those authors did not provide any information on its cell type-specific changes. In pregnant mice, Hgf mRNA is primarily localized to the mesometrial deciduas during Embryonic Days 6.5–8.5, whereas MET protein is present in trophoblast cells [2]. Yang and Park [12] demonstrated that Met mRNA is present in the luminal epithelium of mouse endometrium, but those authors did not specify the estrous stage of the uterus. Yang and Park also detected mouse uterine Hgf mRNA by PCR analysis but failed to localize it by in situ hybridization (ISH) because of the low expression levels. The cyclic changes of HGF and MET expression in mouse uterus and the regulation by ovarian steroids remain unknown.
Secreted as a single-chain zymogen, HGF is cleaved between Arg494 and Val495 to generate a biologically active, disulfide-linked heterodimer [13, 14]. Several potential activators for HGF have been identified that include HGF activator (HGFA), factor XIIa, MT-SP1/matriptase, hepsin, urokinase-type plasminogen activator, and tissue-type plasminogen activator [15–21]. Among them, HGFA exhibits the most potent activity in processing pro-HGF to active HGF [22]. HGFA is produced as an inactive zymogen, the nucleotide sequence of which is homologous to that of coagulation factor XII [19], and it can be activated by thrombin [23]. In addition to the liver, which produces the highest level of HGFA, this protease is also expressed in the kidney [24], brain [25], placenta [26], gastrointestinal tract [27], synovial tissue [28], and hair follicles [29]. Accumulated evidence indicates that HGFA plays an important role in organogenesis, tissue repair, and tumor growth through activating pro-HGF [30–35]. HGFA has been detected in human placental villi and membranes as well as in the decidual cells, suggesting its role in the activation of pro-HGF at these sites [26], but the hormonal regulation of HGFA and its message during pregnancy are unknown. The expression and regulation of HGFA and its message in cycling uterus have not been reported in any species.
The present study was designed to characterize the expression profiles of HGF, MET, and HGFA and their mRNAs in the uterus during the estrous cycle in mice and to explore estrogen and P4 regulation of their expression. In addition, MKI67 immunostaining was conducted to examine the association between HGF/MET expression and endometrial cell proliferation.
MATERIALS AND METHODS
Animals and Steroid Hormone Treatments
CFR/CD1 female mice (age, 8–12 wk) were utilized for all studies. Mice were purchased from Harlan and housed within environmentally controlled conditions under the supervision of a licensed veterinarian. All animal procedures were approved by the University of Kansas Medical Center Institutional Animal and Use Committee. Mice were maintained on a 14L:10D photoperiod and provided water and mice chow ad libitum.
Vaginal smears were used to stage the estrous cycle [36]. Mice exhibiting normal estrous cycles were killed at proestrus, estrus, metestrus, or diestrus between 0900 and 1300 h. To investigate the effects of ovarian steroids, 8-wk-old female mice were ovariectomized and rested for 14 days. Mice were then primed with two daily s.c. injections (Day 1 and Day 2) of 100 ng of 17-beta-estradiol (E2; Sigma) in sesame seed oil before the experiment [37, 38]. On Day 8, groups of three mice were killed by cervical dislocation at 2, 4, 8, and 24 h after one of the following treatments: 1) no treatment (Veh), 2) 4 days (Days 5–8) of daily s.c. injections of 1 mg of P4 in 0.1 ml of sesame seed oil (P4), 3) one (Day 8) s.c. injection of 50 ng of E2 in 0.1 ml of sesame seed oil (E2), 4) 4 days (Days 5–8) of daily s.c. injections of 1 mg of P4 with one s.c. injection of 50 ng of E2 at the same time as the last P4 injection (P+E). The hormonal treatment strategy is well-established and has been used to recapitulate the cell-cycle kinetics of the estrous cycle and early pregnancy [37, 38]. To examine whether the estrogen and P4 regulation of the HGF system is mediated via their corresponding nuclear receptors, mice were injected s.c. with the estrogen-receptor antagonist ICI-182,780 (Tocris Cookson, Inc.; 500 μg/mouse; dissolved in 100% ethanol and resuspended in sesame oil; one injection 30 min before E2 treatment) or the P4-receptor antagonist RU486 (Mifepristone; Tocris Cookson, Inc.; 500 μg/mouse; dissolved in 100% ethanol and resuspended in sesame oil; four daily injections 30 min before P4 treatment) before hormonal treatments for 8 or 24 h. Uteri were removed, snap-frozen in liquid nitrogen, and stored at −80°C until utilized for protein or RNA extraction. For immunohistochemistry and ISH, the middle half of the uterine horn was prepared.
Real-Time Quantitative RT-PCR
Real-time quantitative RT-PCR (qPCR) analysis was carried out as previously described [39]. Total RNA was extracted from frozen uteri using TRIzol reagent. Complementary DNAs were synthesized from 2 μg of total RNA using Maloney murine leukemia virus reverse transcriptase and random hexamer primers. The resulting cDNAs were diluted 1:10 in sterile water, and 1-μl aliquots were used in the qPCR reactions. Primers used were as follows: Hgf (NM_010427), 1227F5′-ACTGACCCAAACATCCGAGTTG-3′ and 1315R5′-TTCCCATTGCCACGATAACAA-3′; Hgfac (NM_019447), 1004F5′-TGCCGGAACCCAGACAAG-3′ and 1090R5′-GGCTGTCAGGCGGCAATA-3′; Met (NM_008591), 1816F5′-AACGAGAGCTGTACCTTGACCTTAA-3′ and 1887R5′-CATCGCGGGACCAACTGT-3′. The qPCR was carried out on an Applied Biosystems HT7900 Sequence Detector. A no-template reaction was included during each experiment to control for DNA contamination. Amplification of 18S rRNA (with Eukaryotic 18S rRNA Endogenous Control, catalog no. 4310893E; AB Applied Biosystems) was used to normalize the level of mRNA expression. The conditions for the real-time qPCR reactions were 50°C for 2 min (one cycle), 95°C for 10 min (one cycle), 95°C for 15 sec and 60°C for 1 min (40 cycles). A standard curve was run in each assay, with an arbitrary value assigned to the highest standard and corresponding values to the subsequent dilutions. Each cDNA sample was run in triplicate. The relative abundance of each target was normalized against that of 18S rRNA.
In Situ Hybridization
In situ hybridization was performed as previously described [40]. Briefly, the middle half of the right uterine horn was excised and embedded in O.C.T. tissue freezing medium (Sakura Fineteck U.S.A., Inc.) and snap-frozen on dry ice. Cryostat sections (thickness, 10 μm) were placed on Fisherbrand Superfrost Plus slides and stored at −80°C until utilized. The cRNA probes were synthesized using the PCR method and digoxigenin (DIG) labeling [41]. Primers were designed incorporating T7 or T3 RNA polymerase promoters in the 5′ end of the primers. DIG-labeled antisense and sense cRNA probes were made by in vitro transcription using the MAXIscript T7/T3 transcription kit (catalog no. 1326; Ambion) and DIG-11-UTP (catalog no. 11209256910; Roche). Prehybridization was performed at 68°C for 1 h in the hybridization mixture (50% formamide, 5× SSC [1× SSC: 0.15 M sodium chloride and 0.015 M sodium citrate], and 120 μg/ml of salmon sperm DNA). After denaturing the probes for 5 min at 80°C, hybridization was performed at 68°C for 1 h and then cooled to 42°C for 20 h. Slides were then treated with 20 μg/ml of RNase A for 30 min at 37°C. After washing in 0.5× SSC for 30 min at 42°C and then in 0.5× SSC for 30 min at room temperature, hybridization signals were detected using an anti-DIG-alkaline phosphatase antibody (catalog no. 11093274910; Roche). The color development was achieved by incubation with 20 μl/ml of BCIP/NBT stock solution (catalog no. 11681451001; Roche), 100 mM NaCl, 50 mM MgCl2 (pH 9.5), and 0.1 M levamisole for 48 h at room temperature in the dark.
Western Blot Analysis
Total protein content was extracted from frozen uteri using radioimmuno-precipitation assay buffer (20 mM Tris [pH 7.5], 150 mM NaCl, 1% IGEPAL CA-630, 0.5% sodium deoxycholate, 1 mM ethylenediaminetetra-acetic acid, and 0.1% SDS) containing a protease/phosphatase inhibitor cocktail (0.1 mg/ml PMSF, 30 μl/ml of aprotinin, 5 μg/ml of leupeptin, and 1 mM sodium orthovanadate; Sigma). Protein concentrations were determined using the DC Protein Assay (Bio-Rad Laboratories). Thirty-five micrograms of proteins were subjected to 4–12% Bis-Tris (Invitrogen) gel electrophoresis and electroblotted onto polyvinylidene fluoride membranes (Invitrogen). After blocking with 5% nonfat dry milk in Tris-buffered saline Tween-20 (50 mM Tris-HCl, 150 mM NaCl, and 0.05% Tween-20, pH 7.5) for 2 h at room temperature, blots were incubated with 0.2 μg/ml of goat-anti-mouse HGF-α (AF2207; R&D Systems) or 0.6 μg/ml of goat-anti-HGFA-L (N-19, sc-1371; Santa Cruz Biotechnology, Inc.) overnight at 4°C. On the second day, blots were washed and incubated with rabbit-anti-goat-HRP (Jackson ImmunoResearch Laboratories, Inc.) for 1 h at room temperature. Blots were then washed and immunodetection conducted using an enhanced chemiluminescence (ECL) kit (Amersham Biosciences). Next, blots were stripped and reprobed for β-actin using goat-anti-β-actin (I-19, sc-1616; Santa Cruz Biotechnology). The expression level of each protein was normalized to the level of β-actin.
Immunohistochemistry
Uterine specimens fixed in 10% neutral buffered formalin and embedded in paraffin wax (section thickness, 5 μm) were deparaffinized with xylene and rehydrated with a graded series of ethanol. Antigen retrieval was conducted using antigen-unmasking solution (H-3300; Vector Laboratories, Inc.). Sections were incubated in 3% H2O2 in water for 10 min for quenching of endogenous peroxidase activity. Immunohistochemistry was carried out using the VECTASTAIN elite ABC Kits (PK6105 for goat IgG and PK6101 rabbit IgG; Vector Laboratories, Inc.). After blocking with appropriate normal serum, sections were incubated with primary antibodies overnight at 4°C. On the second day, slides were washed and incubated with appropriate secondary antibodies for 30 min at room temperature. Slides were then incubated with ABC reagents for 30 min at room temperature, stained with a diaminobenzidine substrate kit (SK-4100; Vector Laboratories, Inc.), and briefly counterstained with hematoxylin. Primary antibodies used were as follows: goat-anti-HGFA-L (1 μg/ml, N-19, sc-1371; Santa Cruz Biotechnology), rabbit-anti-HGFα (0.5 μg/ml, 18281; IBL Co.), rabbit-anti-MET (0.125 μg/ml, sc-162; Santa Cruz Biotechnology), and goat-anti-MKI67 (0.06 μg/ml, M-19, sc-7846; Santa Cruz Biotechnology). Substitution of the primary antibodies with appropriate polyclonal IgG was used as a negative control. Mouse lung or kidney was used as a positive control.
Statistical Analysis
All data were analyzed using SPSS Version 13.0 software (SPSS, Inc.). ANOVA was used for comparison across treatment regimes. When an F test indicated statistical significance, post hoc analysis was made using the Tukey honestly significant difference procedure. Significance was set at P < 0.05 for all comparisons.
RESULTS
HGF and Hgf
Cycling uteri.
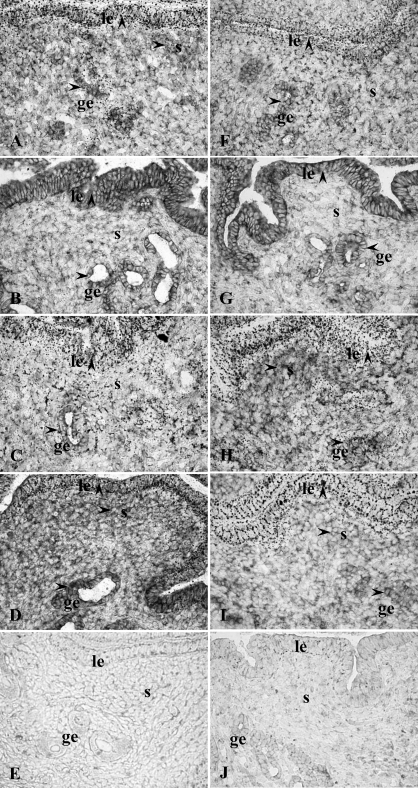
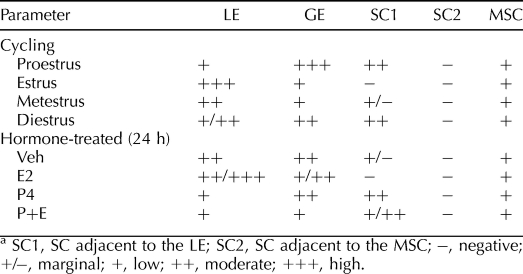
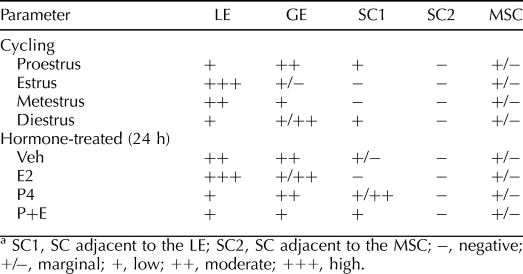
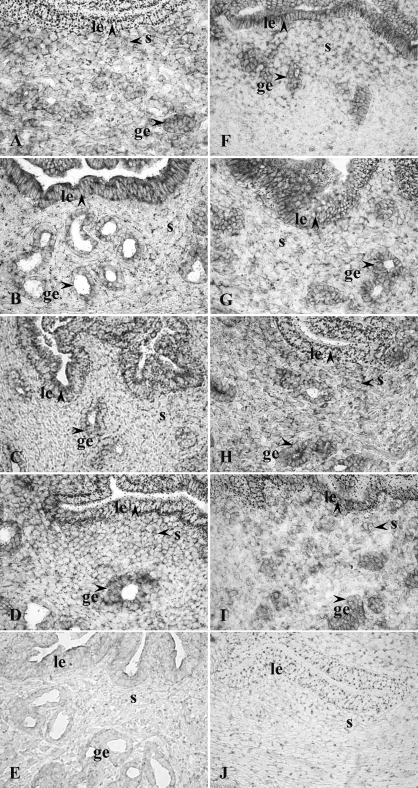
HGF protein was primarily localized to glandular and luminal epithelial cells as well as stromal cells (SC) in cycling uteri (Fig. 1, A–E, and Table 1). HGF immunoreactivity was observed in SC adjacent to the luminal epithelium (LE) at diestrus and proestrus, whereas minimal stromal staining was observed at estrus and metestrus. A marked decrease in HGF immunoreactivity in GE and a marked increase at the apical side of the LE were observed at estrus. Faint HGF expression was also detected in myometrial smooth muscle cells (MSC) across the estrous cycle (data not shown). In the lung (Supplemental Fig. S1; all supplemental data are available online at www.biolreprod.org), HGF immunoreactivity was observed in airway epithelia.
FIG. 1.
Immunohistochemistry of HGF protein expression in cycling uteri (A–E) and steroid-treated uteri (F–O): A, proestrus; B, estrus; C, metestrus; D, diestrus; E, estrus, negative control; F, Veh, 24 h; G, E2, 24 h; H, P4, 24 h; I, P+E, 24 h; J, P4, 24 h, negative control; K, E2 plus ICI-182,780 (ICI), 24 h; L, P4 plus RU486 (RU), 24 h; M, P+E plus ICI, 24 h; N) P+E plus RU, 24 h; O) P+E plus ICI, 24 h, negative control. Arrowhead indicates positive immunostaining. ge, glandular epithelium; le, luminal epithelium; s, stroma. Orginal magnification ×400.
TABLE 1.
Semi-quantitative analysis of HGF IHC.a
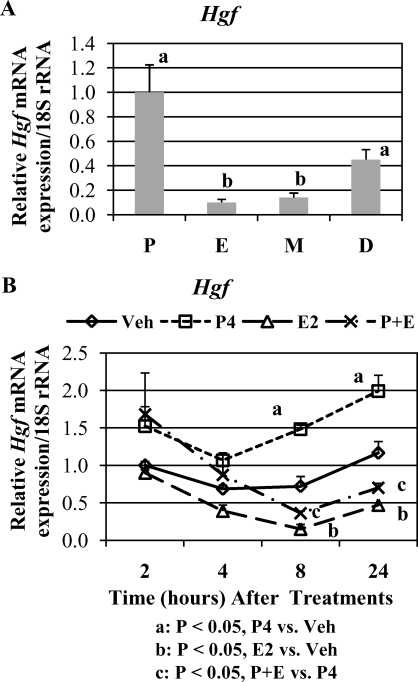
Western blot analysis demonstrated that HGF protein was in mature form across the cycle. Uterine HGF protein levels were high at proestrus, drastically decreased at estrus, remained low at metestrus, and increased again at diestrus stage (Fig. 2).
FIG. 2.
Western blot analysis of HGF protein expression in cycling uteri (n = 5 mice/estrous stage). Different lowercase letters indicate statistical significance (one-way ANOVA, P < 0.05). D, diestrus; E, estrus; M, metestrus; P, proestrus.
The ISH analysis revealed an expression pattern of Hgf mRNA similar to that of HGF protein (Fig. 3, A–E, and Table 2). Stromal staining was observed in SC adjacent to the LE at diestrus and proestrus but not at estrus and metestrus. At estrus, Hgf mRNA expression was decreased in the GE and increased in LE. Please note that some nonspecific signals were caused by lipid vesicles in and around the LE on some sections (these nonspecific signals were not observed at estrus stage or after estrogen treatment).
FIG. 3.
ISH analysis of Hgf mRNA expression: A) proestrus; B) estrus; C) metestrus; D) diestrus; E) proestrus, sense probe; F) Veh, 24 h; G) E2, 24 h; H) P4, 24 h; I) P+E, 24 h; J) E2, 24 h, sense probe. Arrowhead indicates positive staining. ge, glandular epithelium; le, luminal epithelium; s, stroma. Original magnification ×400.
TABLE 2.
Semi-quantitative analysis of Hgf ISH.a
The qPCR analysis indicated that steady-state levels of Hgf mRNA paralleled those of HGF protein in cycling uterus (Fig. 4A).
FIG. 4.
qPCR analysis of Hgf mRNA expression. mRNA levels are expressed as mean ratio of mRNA to 18S rRNA ± SEM. A) Cycling uteri (n = 5 mice/estrous stage). Different lowercase letters indicate statistical significance among the treatment groups (one-way ANOVA, P < 0.05). B) Steroid-treated uteri (n = 3 mice/treatment group/time point).
Steroid-treated uteri.
Immunohistochemistry showed that uterine HGF protein (Fig. 1, F–O, and Table 1 and Supplemental Fig. S2) was primarily localized to LE and GE in the Veh group. P4 decreased HGF expression in the LE and induced it in the SC adjacent to the LE. E2 decreased HGF staining in the epithelial cells of some, but not all, endometrial glands and induced a shift of luminal epithelial HGF protein to the apical surface at 24 h. The HGF staining in the GE without a lumen remained high in the 24-h E2 group. In the P+E group, the HGF staining was decreased in both GE and LE but increased in SC adjacent to the LE at 24 h as compared to the Veh group. Compared with P4 alone, cotreatment with E2 and P4 decreased HGF staining intensity in the GE. Faint HGF expression was also detected in the MSC in each treatment group without apparent differences. Pretreatment with ICI-182,780 or RU486 blocked the effects of E2 and P4, respectively, at 24 h.
The ISH analysis revealed that Hgf mRNA expression pattern was consistent with that of HGF protein in steroid-treated uteri (Fig. 3, F–J, and Table 2). P4 induced subepithelial stromal Hgf mRNA expression but decreased its expression in the LE. Hgf mRNA expression was decreased in some of the endometrial glands at 24 h in the E2 group. In the P+E group, Hgf mRNA expression was decreased in all the glands.
The qPCR analysis (Fig. 4B) demonstrated that Hgf steady-state mRNA levels were increased by P4 but decreased by E2. In addition, E2 inhibited/reversed P4-induced up-regulation of Hgf mRNA.
HGFA and Hgfac
Cycling uteri.
The expression of HGFA protein was primarily localized to the GE, LE, glandular lumen, and the SC adjacent to the LE (Fig. 5, A–E, and Table 3). HGFA immunoreactivity in the GE was highest at proestrus and lowest at estrus and metestrus. Luminal epithelial expression of HGFA was slightly increased at the apical side at estrus. At diestrus and proestrus, HGFA was also present in SC adjacent to the LE. Minimal staining was observed in the MSC. In the kidney, HGFA immunoreactivity was observed in the epithelial cells of proximal and distal convoluted tubules (Supplemental Fig. S3).
FIG. 5.
Immunohistochemistry of HGFA protein expression in cycling uteri (A–E) and steroid-treated uteri (F–O): A) proestrus; B) estrus; C) metestrus; D) diestrus; E) proestrus, negative control; F) Veh, 24 h; G) E2, 24 h; H) P4, 24 h; I) P+E, 24 h; J) P4, 24 h, negative control; K) E2 plus ICI-182,780 (ICI), 24 h; L) P4 plus RU486 (RU), 24 h; M) P+E plus ICI, 24 h; N) P+E plus RU, 24 h; O) P+E plus RU, 24 h, negative control. Arrowhead indicates positive immunostaining. ge, glandular epithelium; le, luminal epithelium; s, stroma. Original magnification ×400.
TABLE 3.
Semi-quantitative analysis of HGFA IHC.a
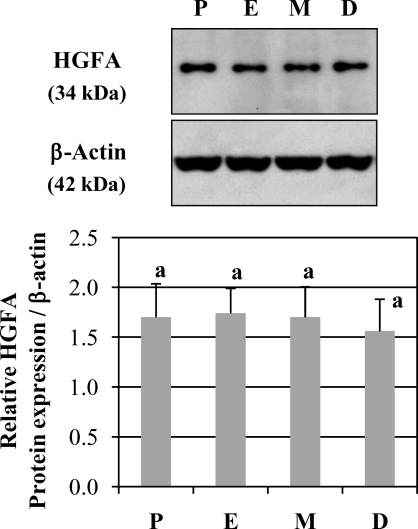
Western blot analysis detected HGFA protein at 34 kDa, which represents the active form of this protease. No significant differences in HGFA protein levels were observed in homogenized uteri (Fig. 6).
FIG. 6.
Western blot analysis of HGFA protein expression in cycling uteri (n = 5 mice/estrous stage). No significant difference was observed (one-way ANOVA, P > 0.05). D, diestrus; E, estrus; M, metestrus; P, proestrus.
Hgfac mRNA expression was assessed by ISH (Fig. 7, A–E, and Table 4). At proestrus, Hgfac mRNA was primarily localized to the GE, SC adjacent to the LE, and to a lesser extent, LE. At estrus, its expression was increased in the LE but decreased in GE and disappeared in SC. At metestrus, Hgfac mRNA expression levels in LE and GE were similar. At diestrus, Hgfac mRNA reappeared in the SC adjacent to the LE.
FIG. 7.
ISH analysis of Hgfac mRNA expression: A) proestrus; B) estrus; C) metestrus; D) diestrus; E) estrus, sense probe; F) Veh, 24 h; G) E2, 24 h; H) P4, 24 h; I) P+E, 24 h; J) E2, 24 h, sense probe. Original magnification ×400. Arrowhead indicates positive staining. le, luminal epithelium; ge, glandular epithelium; s, stroma.
TABLE 4.
Semi-quatitative analysis of Hgfac ISH.a
The qPCR analysis (Fig. 8A) revealed that uterine Hgfac steady-state mRNA levels were low at diestrus and proestrus. As estrus, Hgfac mRNA levels were increased by 20-fold (P < 0.01) as compared to proestrus.
FIG. 8.
qPCR analysis of Hgfac mRNA expression. mRNA levels are expressed as mean ratio of mRNA to 18S rRNA ± SEM. A) Cycling uteri (n = 5 mice/estrous stage). Different letters indicate statistical significance among the treatment groups (one-way ANOVA, P < 0.05). B) Steroid-treated uteri (n = 3 mice/treatment group/time point).
Steroid-treated uteri.
HGFA protein (Fig. 5, F–O, and Table 3 and Supplemental Fig. S4) was localized to the endometrial LE and GE, with higher intensity on the apical surface in ovariectomized mice (Veh group). In the P4 group, HGFA in LE was evenly distributed in the cells instead of on the apical side. P4 also induced HGFA expression in the SC adjacent to the LE. The HGFA expression after E2 treatment was similar to that in the Veh group at 24 h. Compared with P4 alone, cotreatment with E2 and P4 decreased HGFA immunoreactivity in the GE at 24 h. At 8 h, E2, either alone or used with P4, also induced HGFA staining in the SC adjacent to the myometrium (Supplemental Fig. S4). Pretreatment with ICI-182,780 or RU486 blocked the effects of E2 and P4, respectively, at 24 h (Fig. 5, F–O) and 8 h (Supplemental Fig. S4).
The ISH revealed that Hgfac mRNA was primarily localized to the GE and LE in the Veh groups. After E2 treatment for 24 h, its abundance in the LE was significantly increased. P4 induced its expression in the SC adjacent to the LE and decreased it in the LE. Compared with P4 alone, cotreatment with E2 and P4 decreased Hgfac mRNA expression in the GE at 24 h (Fig. 7, F–J). At 8 h (Supplemental Fig. S5), E2, either used alone or with P4, did not significantly affect Hgfac expression as compared to the Veh or P4 group.
The qPCR analysis revealed that E2 induced an increase in total uterine Hgfac steady-state mRNA levels at 24 h (P < 0.05). P4 alone showed no significant effects on total uterine Hgfac mRNA levels, but pretreatment with P4 blocked E2-up-regulation of Hgfac mRNA (Fig. 8B).
MET and Met
Cycling uteri.
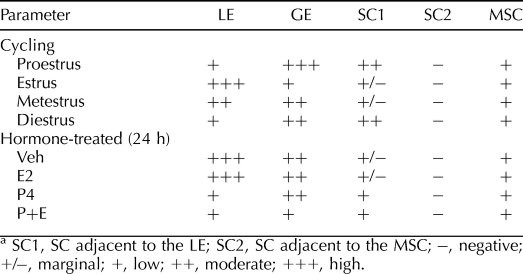
Overall, the expression of MET protein in the cycling uteri showed a pattern similar to that of HGF (Fig. 9, A–E, and Table 5). MET immunoreactivity was decreased in the GE but increased in the LE, especially on the apical surface at estrus. At diestrus and proestrus, SC adjacent to the LE was also positively stained for MET. Faint staining for MET was also observed in MSC and endothelial cells in the myometrium across the estrous cycle, with no significant variations. In the lung, MET immunoreactivity was observed in airway epithelia (Supplemental Fig. S6).
FIG. 9.
Immunohistochemistry of MET protein expression in cycling uteri (A–E) and steroid-treated uteri (F–O): A) proestrus; B) estrus; C) metestrus; D) diestrus; E) diestrus, negative control; F, Veh, 24 h; G) E2, 24 h; H) P4, 24 h; I) P+E, 24 h; J) P4, 24 h, negative control; K) E2 plus ICI-182,780 (ICI), 24 h; L) P4 plus RU486 (RU), 24 h; M) P+E plus ICI, 24 h; N) P+E plus RU, 24 h; O) E2 plus ICI, 24 h, negative control. Arrowhead indicates positive immunostaining. ge, glandular epithelium; le, luminal epithelium; s, stroma. Original magnification ×400.
TABLE 5.
Semi-quantitative analysis of MET IHC.a
The ISH analysis revealed that cellular expression pattern of Met mRNA was consistent with that of MET protein (Fig. 10, A–E, and Table 6). At estrus, Met mRNA expression was decreased in the GE but increased in the LE. Stromal staining was observed in the SC adjacent to the LE at diestrus and proestrus but not at estrus and metestrus.
FIG. 10.
ISH analysis of Met mRNA expression: A) proestrus; B) estrus; C) metestrus; D) diestrus; E) estrus, sense probe; F, Veh 24 h; G) E2, 24 h; H) P4, 24 h; I) P+E, 24 h, J) P4, 24 h, sense probe. Arrowhead indicates positive staining. ge, glandular epithelium; le, luminal epithelium; s, stroma. Original magnification ×400.
TABLE 6.
Semi-quantitative analysis of Met ISH.a
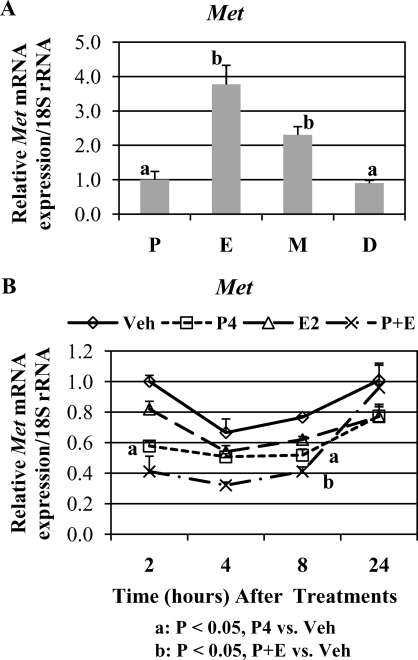
The qPCR analysis demonstrated that Met mRNA levels showed moderate variations across the estrous cycle (Fig. 11A). Highest Met mRNA levels were shown at estrus. At diestrus and proestrus, total uterine Met mRNA levels were roughly one third of those at estrus (P < 0.05).
FIG. 11.
qPCR analysis of Met mRNA expression. The mRNA levels are expressed as mean ratio of mRNA to 18S rRNA ± SEM. A) Cycling uteri (n = 5 mice/estrous stage). Different letters indicate statistical significance among the treatment groups (one-way ANOVA, P < 0.05). B) Steroid-treated uteri (n = 3 mice/treatment group/time point).
Steroid-treated uteri.
Uterine MET protein was primarily localized to endometrial GE and LE in the Veh group. P4 decreased MET expression in the LE but induced it in the SC adjacent to the LE. E2 did not significantly affect MET expression. However, compared with P4 alone, cotreatment with E2 and P4 decreased MET expression in the GE at 24 h. As compared to the Veh group, cotreatment with E2 and P4 decreased MET expression was decreased in both the GE and the LE but induced in the SC at 24 h. Low levels of MET were also detected in the MSC of uteri from each treatment group without apparent differences. Pretreatment with ICI-182,780 or RU486 blocked the effects of E2 and P4, respectively, at 24 h (Fig. 9, F–O, and Table 5 and Supplemental Fig. S7).
The ISH analysis revealed that Met mRNA expression pattern agreed with MET protein in steroid-treated uteri. P4 induced subepithelial stromal Met mRNA expression but decreased its expression in the LE. Compared with P4 alone, cotreatment with E2 and P4 decreased Met mRNA expression in the GE at 24 h (Fig. 10, F–J, and Table 6).
The qPCR analysis revealed that E2 had no significant effect on total uterine Met mRNA levels, whereas P4 or cotreatment with E2 and P4 slightly but significantly decreased Met mRNA levels (Fig. 11B).
MKI67
Cycling uteri.
MKI67 immunohistochemistry was conducted as a marker of cell proliferation. Results showed that MKI67 was primarily localized to the GE, LE, as well as SC in cycling uteri (Fig. 12). At diestrus and proestrus, nuclear MKI67 staining in SC was primarily localized to those adjacent to the LE. At estrus, although some MKI67-positive nuclei were randomly distributed throughout the stroma, the number of positively stained SC adjacent to the LE was markedly reduced. At metestrus, few SC were positively stained for MKI67. Moreover, nuclear MKI67 expression was decreased in the GE at estrus as compared to diestrus and proestrus.
FIG. 12.
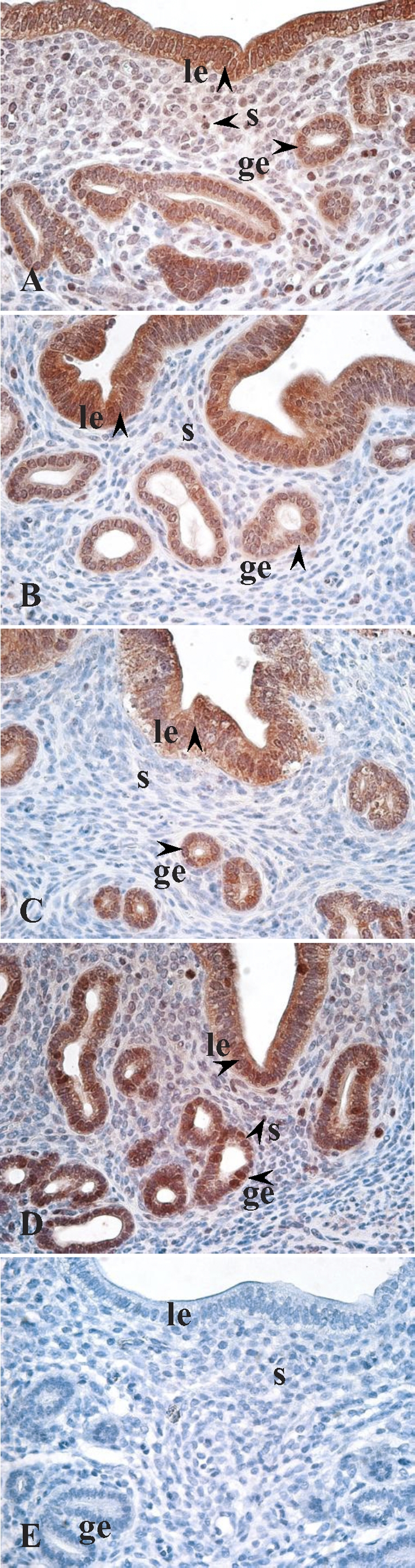
Immunohistochemistry of MKI67 protein expression in cycling uteri (A–E): A) proestrus; B) estrus; C) metestrus; D) diestrus; E) proestrus, negative control. Arrowhead indicates positive immunostaining. ge, glandular epithelium; le, luminal epithelium; s, stroma. Original magnification ×400.
DISCUSSION
Findings from the present study indicate a cell type- and compartment-specific regulation of the uterine HGF system by E2 and P4 during the estrous cycle. Serum E2 and P4 levels vary across the estrous stages. E2 levels are highest at estrus, whereas P4 levels are highest at diestrus [42, 43]. The increase in HGF, HGFA, and MET expression in the SC adjacent to the LE at diestrus appears to be a P4 response. The increase in LE expression of HGF and MET at estrus appears to be the result of decreased P4 levels. The increase in LE Hgfac mRNA expression at estrus perhaps results from both decreased P4 and increased E2 levels. LE expression of HGFA protein only showed a slight increase at the apical side at estrus. Western blot analysis failed to show any differences in HGFA protein levels in homogenized uteri across the cycle. The reason for the disparity between HGFA protein and mRNA is not understood. One possible explanation is that the luminal epithelial cells release HGFA into the uterine lumen. This is supported by the demonstration of HGFA immunostaining in the lumen of some glands, especially at proestrus and estrus. Alternatively, posttranscriptional and/or posttranslational modifications may be involved in the regulation of uterine HGFA expression. It is equally possible that there are some isoforms of HGFA or its precursor in the mouse uterus that the antibody used in the present study failed to interact in immunostaining and Western blot analysis. The decrease in HGF, MET, and HGFA expression in the GE at estrus appears to be an E2 response, but P4 priming is necessary for E2 to fully exert its effects. Pretreatment with ICI-182,780 or RU486 blocked the effects of E2 and P4, respectively, indicating that the hormonal effects are mediated through their cognate nuclear receptors.
The co-ordinated and sequential actions of estrogen and P4 direct uterine tissue remodeling during estrous cycling while preparing for a receptive endometrium for blastocyst implantation. The dynamic hormonal regulation of the HGF system in the mouse uterus likely plays a critical role in uterine remodeling during both the estrous cycle and pregnancy by constantly altering endometrial anatomy and physiology in a cell type- and compartment-specific manner. HGF-induced disruption of epithelial cell junctions and increase in paracellular permeability are implicated in the dissociation of cancer cells [44]. At diestrus, increased HGF expression in the GE would likely increase the permeability of cell junctions, leading to increased secretion of histotroph nutrients required for peri-implantation blastocyst development. Junctional complex integrity plays a role in trophoblast attachment to the LE [45]. The P4 down-regulation of HGF expression in the LE would theoretically facilitate preimplantation embryo attachment on the LE. Treatment of pregnant animals in the preimplantation stage with RU486 blocks implantation [46]. It would be interesting to examine if the effect of RU486 in blocking the P4-induced decrease in HGF expression in the LE contributes to this phenomenon.
The pattern of glandular epithelial cell proliferation differs from that of luminal epithelial cells [47]. During the estrous cycle, luminal epithelial cell proliferation does not significantly change, but the glandular epithelial cell proliferation is significantly decreased at estrus [48] (present study). Wood et al. [43] reported that circulating P4 levels inversely correlated with uterine luminal epithelial proliferation but that E2 levels inversely correlated with glandular proliferation in cycling uteri. The present study showed a positive correlation between HGF/MET expression and MKI67 immunoreactivity in the mouse endometrium during the estrous cycle. Because HGF is a well-known mitogen, it seems reasonable to postulate that HGF/MET may be involved in the compartment-specific regulation of endometrial cell proliferation by ovarian steroid hormones. The compartment-specific effects of the estrous cycle on the expression of the uterine HGF system are most probably caused not only by levels of E2 and P4 but also by their compartment-specific effects on the expression of estrogen and P4 receptors [48].
The cellular expression of HGF and MET in the endometrium seems to be both species-specific and developing stage-specific. The differences in their expression patterns between the mouse and the sheep are interesting and, perhaps, are related to the different implantation schemes. Although Met mRNA was previously localized only to mouse endometrial luminal epithelial cells [12], those authors did not specify the estrous stage of the uterus. Based on the morphology, it was most likely at estrus. As shown in the present study, Met mRNA was primarily localized to endometrial epithelial cells at estrus, with much higher intensity in the LE than in the GE. The origin of HGF mRNA in human endometrium remains elusive. Although HGF protein has been localized to both epithelial cells and SC in human endometrium in vivo, Sugawara et al. [5] failed to detect HGF mRNA expression in isolated human endometrial epithelial cells. Data from the present study indicate that both endometrial epithelial cells and SC have the capacity to express HGF and MET proteins and their mRNAs in murine uterus. Epithelial localization of HGF/Hgf mRNA in other organs has been reported previously [49–52]. The concept that HGF is a stromal-derived growth factor is mainly based upon in vitro cell culture studies. It is noteworthy that the cell type-specific expression pattern of HGF may change during in vitro cell culture [53].
Hepatocyte growth factor is not only a mitogen but also a motogen and a morphogen. In the present study, a gradient of HGF concentration in both the LE and the SC was noted. The role of this gradient is not clear at this point, but a few possibilities exist. During tissue repair after injures, increased HGF expression is found at the leading edge of the epithelial cells, which triggers epithelial cell migration and, thus, healing of the epithelium. At estrus, increased HGF expression was observed at the edge of the LE, suggesting a potential role for HGF in luminal epithelial cell migration and reorganization. It is also possible that the gradient of HGF in the stroma may function as a chemoattractant and induce cell migration in the stroma toward the LE.
The expression of HGFA protein, but not its mRNA, has recently been shown in the rat ovary [54]. The murine uterus, however, is capable of synthesizing both HGFA protein and its mRNA. Although the present study does not show any direct evidence of HGF activation by HGFA in the mouse uterus, the correlation between the expression pattern of HGFA and those of HGF/MET suggests that HGFA perhaps play a role in uterine HGF activation. Interestingly, increased HGFA protein expression was observed in the stroma at 8 h following E2 treatment, whereas no parallel up-regulation of Hgfac mRNA was observed. The E2 induction of stromal HGF expression was blocked by ICI-182,780 pretreatment, indicating a nuclear estrogen receptor effect. This up-regulation is not likely to result from either de novo synthesis or release from the epithelial cells. Most likely, it results from infiltration from the bloodstream. The liver produces the highest amount of pro-HGFA and releases it into the plasma. Estrogen has been shown to induce uterine uptake of plasma proteins, such as albumin, plasminogen, and prothrombin [55, 56]. In cycling uteri, HGFA is not expressed in the stroma adjacent to the myometrium. HGFA expression in peri-implantation uteri has not been examined. As such, it is currently unknown whether the up-regulation of HGFA protein in the stroma by E2 at 8 h has any physiological association.
In summary, data obtained from the present study provide, to our knowledge, the first evidence that HGF, HGFA, and MET expression in mouse uterus changes during the estrous cycle in a stage-, cell type-, and compartment-specific manner under the influence of estrogen and P4. The findings provide a foundation for further investigation on the role of the HGF system in mediating hormonal regulation of uterine biology.
Acknowledgments
The author is grateful to Dr. Thomas Spencer for his advice concerning this study and to Dr. Warren Nothnick for offering free access to his laboratory.
Footnotes
Supported by grant award HD055249 from the NICHD/NIH to X.Z. Presented in part at the 42nd Annual Meeting of the Society for the Study of Reproduction, July 18–22, 2009, Pittsburgh, Pennsylvania.
REFERENCES
- Takayama H, LaRochelle WJ, Sharp R, Otsuka T, Kriebel P, Anver M, Aaronson SA, Merlino G.Diverse tumorigenesis associated with aberrant development in mice overexpressing hepatocyte growth factor/scatter factor. Proc Natl Acad Sci U S A 1997; 94: 701–706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel Y, Kim H, Rappolee DA.A role for hepatocyte growth factor during early postimplantation growth of the placental lineage in mice. Biol Reprod 2000; 62: 904–912. [DOI] [PubMed] [Google Scholar]
- Spencer TE, Bazer FW.Biology of progesterone action during pregnancy recognition and maintenance of pregnancy. Front Biosci 2002; 7: d1879–d1898. [DOI] [PubMed] [Google Scholar]
- Taylor KM, Chen C, Gray CA, Bazer FW, Spencer TE.Expression of messenger ribonucleic acids for fibroblast growth factors 7 and 10, hepatocyte growth factor, and insulin-like growth factors and their receptors in the neonatal ovine uterus. Biol Reprod 2001; 64: 1236–1246. [DOI] [PubMed] [Google Scholar]
- Sugawara J, Fukaya T, Murakami T, Yoshida H, Yajima A.Hepatocyte growth factor stimulated proliferation, migration, and lumen formation of human endometrial epithelial cells in vitro. Biol Reprod 1997; 57: 936–942. [DOI] [PubMed] [Google Scholar]
- Yoshida S, Harada T, Iwabe T, Taniguchi F, Fujii A, Sakamoto Y, Yamauchi N, Shiota G, Terakawa N.Induction of hepatocyte growth factor in stromal cells by tumor-derived basic fibroblast growth factor enhances growth and invasion of endometrial cancer. J Clin Endocrinol Metab 2002; 87: 2376–2383. [DOI] [PubMed] [Google Scholar]
- Yoshida S, Harada T, Mitsunari M, Iwabe T, Sakamoto Y, Tsukihara S, Iba Y, Horie S, Terakawa N.Hepatocyte growth factor/Met system promotes endometrial and endometriotic stromal cell invasion via autocrine and paracrine pathways. J Clin Endocrinol Metab 2004; 89: 823–832. [DOI] [PubMed] [Google Scholar]
- Wagatsuma S, Konno R, Sato S, Yajima A.Tumor angiogenesis, hepatocyte growth factor, and c-Met expression in endometrial carcinoma. Cancer 1998; 82: 520–530. [DOI] [PubMed] [Google Scholar]
- Chen C, Spencer TE, Bazer FW.Expression of hepatocyte growth factor and its receptor c-met in the ovine uterus. Biol Reprod 2000; 62: 1844–1850. [DOI] [PubMed] [Google Scholar]
- Satterfield MC, Hayashi K, Song G, Black SG, Bazer FW, Spencer TE.Progesterone regulates FGF10, MET, IGFBP1, and IGFBP3 in the endometrium of the ovine uterus. Biol Reprod 2008; 79: 1226–1236. [DOI] [PubMed] [Google Scholar]
- Lindsey JS, Brenner RM.Novel hepatocyte growth factor/scatter factor isoform transcripts in the macaque endometrium and placenta. Mol Hum Reprod 2002; 8: 81–87. [DOI] [PubMed] [Google Scholar]
- Yang XM, Park M.Expression of the hepatocyte growth factor/scatter factor receptor tyrosine kinase is localized to epithelia in the adult mouse. Lab Invest 1995; 73: 483–491. [PubMed] [Google Scholar]
- Gak E, Taylor WG, Chan AM, Rubin JS.Processing of hepatocyte growth factor to the heterodimeric form is required for biological activity. FEBS Lett 1992; 311: 17–21. [DOI] [PubMed] [Google Scholar]
- Naka D, Ishii T, Yoshiyama Y, Miyazawa K, Hara H, Hishida T, Kidamura N.Activation of hepatocyte growth factor by proteolytic conversion of a single chain form to a heterodimer. J Biol Chem 1992; 267: 20114–20119. [PubMed] [Google Scholar]
- Naldini L, Tamagnone L, Vigna E, Sachs M, Hartmann G, Birchmeier W, Daikuhara Y, Tsubouchi H, Blasi F, Comoglio PM.Extracellular proteolytic cleavage by urokinase is required for activation of hepatocyte growth factor/scatter factor. EMBO J 1992; 11: 4825–4833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimomura T, Ochiai M, Kondo J, Morimoto Y.A novel protease obtained from FBS-containing culture supernatant that processes single chain form hepatocyte growth factor to two chain form in serum-free culture. Cytotechnology 1992; 8: 219–229. [DOI] [PubMed] [Google Scholar]
- Shimomura T, Miyazawa K, Komiyama Y, Hiraoka H, Naka D, Morimoto Y, Kitamura N.Activation of hepatocyte growth factor by two homologous proteases, blood-coagulation factor XIIa and hepatocyte growth factor activator. Eur J Biochem 1995; 229: 257–261. [DOI] [PubMed] [Google Scholar]
- Mars WM, Zarnegar R, Michalopoulos GK.Activation of hepatocyte growth factor by the plasminogen activators uPA and tPA. Am J Pathol 1993; 143: 949–958. [PMC free article] [PubMed] [Google Scholar]
- Miyazawa K, Shimomura T, Kitamura A, Kondo J, Morimoto Y, Kitamura N.Molecular cloning and sequence analysis of the cDNA for a human serine protease responsible for activation of hepatocyte growth factor: structural similarity of the protease precursor to blood coagulation factor XII. J Biol Chem 1993; 268: 10024–10028. [PubMed] [Google Scholar]
- Kirchhofer D, Peek M, Lipari MT, Billeci K, Fan B, Moran P.Hepsin activates pro-hepatocyte growth factor and is inhibited by hepatocyte growth factor activator inhibitor-1B (HAI-1B) and HAI-2. FEBS Lett 2005; 579: 1945–1950. [DOI] [PubMed] [Google Scholar]
- Lee SL, Dickson RB, Lin CY.Activation of hepatocyte growth factor and urokinase/plasminogen activator by matriptase, an epithelial membrane serine protease. J Biol Chem 2000; 275: 36720–36725. [DOI] [PubMed] [Google Scholar]
- Kataoka H, Miyata S, Uchinokura S, Itoh H.Roles of hepatocyte growth factor (HGF) activator and HGF activator inhibitor in the pericellular activation of HGF/scatter factor. Cancer Metastasis Rev 2003; 22: 223–236. [DOI] [PubMed] [Google Scholar]
- Shimomura T, Kondo J, Ochiai M, Naka D, Miyazawa K, Morimoto Y, Kitamura N.Activation of the zymogen of hepatocyte growth factor activator by thrombin. J Biol Chem 1993; 268: 22927–22932. [PubMed] [Google Scholar]
- Van Adelsberg J, Sehgal S, Kukes A, Brady C, Barasch J, Yang J, Huan Y.Activation of hepatocyte growth factor (HGF) by endogenous HGF activator is required for metanephric kidney morphogenesis in vitro. J Biol Chem 2001; 276: 15099–15106. [DOI] [PubMed] [Google Scholar]
- Yamada T, Yoshiyama Y, Tsuboi Y, Shimomura T.Astroglial expression of hepatocyte growth factor and hepatocyte growth factor activator in human brain tissues. Brain Res 1997; 762: 251–255. [DOI] [PubMed] [Google Scholar]
- Somerset DA, Strain AJ, Afford S, Whittle MJ, Kilby MD.Hepatocyte growth factor activator (HGF-A) and its zymogen in human placenta. Placenta 2000; 21: 615–620. [DOI] [PubMed] [Google Scholar]
- Kinoshita Y, Kishi K, Asahara M, Matasushima Y, Wang HY, Miyazawa K, Kitamura N, Chiba T.Production and activation of hepatocyte growth factor during the healing of rat gastric ulcers. Digestion 1997; 58: 225–231. [DOI] [PubMed] [Google Scholar]
- Nagashima M, Hasegawa J, Kato K, Yamazaki J, Nishigai K, Ishiwata T, Asano G, Yoshino S.Hepatocyte growth factor (HGF), HGF activator, and c-Met in synovial tissues in rheumatoid arthritis and osteoarthritis. J Rheumatol 2001; 28: 1772–1778. [PubMed] [Google Scholar]
- Lee YR, Yamazaki M, Mitsui S, Tsuboi R, Ogawa H.Hepatocyte growth factor (HGF) activator expressed in hair follicles is involved in in vitro HGF-dependent hair follicle elongation. J Dermatol Sci 2001; 25: 156–163. [DOI] [PubMed] [Google Scholar]
- van Adelsberg J, Sehgal S, Kukes A, Brady C, Barasch J, Yang J, Huan Y.Activation of hepatocyte growth factor (HGF) by endogenous HGF activator is required for metanephric kidney morphogenesis in vitro. J Biol Chem 2001; 276: 15099–15106. [DOI] [PubMed] [Google Scholar]
- Kinoshita Y, Kishi K, Asahara M, Matasushima Y, Wang HY, Miyazawa K, Kitamura N, Chiba T.Production and activation of hepatocyte growth factor during the healing of rat gastric ulcers. Digestion 1997; 58: 225–231. [DOI] [PubMed] [Google Scholar]
- Okajima A, Miyazawa K, Naitoh Y, Inoue K, Kitamura N.Induction of hepatocyte growth factor activator messenger RNA in the liver following tissue injury and acute inflammation. Hepatology 1997; 25: 97–102. [DOI] [PubMed] [Google Scholar]
- Yamauchi M, Kataoka H, Itoh H, Seguchi T, Hasui Y, Osada Y.Hepatocyte growth factor activator inhibitor types 1 and 2 are expressed by tubular epithelium in kidney and down-regulated in renal cell carcinoma. J Urol 2004; 171: 890–896. [DOI] [PubMed] [Google Scholar]
- Tjin EP, Derksen PW, Kataoka H, Spaargaren M, Pals ST.Multiple myeloma cells catalyze hepatocyte growth factor (HGF) activation by secreting the serine protease HGF-activator. Blood 2004; 104: 2172–2175. [DOI] [PubMed] [Google Scholar]
- Kataoka H, Itoh H, Hamasuna R, Meng JY, Koono M.Pericellular activation of hepatocyte growth factor/scatter factor (HGF/SF) in colorectal carcinomas: roles of HGF activator (HGFA) and HGFA inhibitor type 1 (HAI-1). Hum Cell 2001; 14: 83–93. [PubMed] [Google Scholar]
- Nelson JF, Felicio LS, Randall PK, Sims C, Finch CE.A longitudinal study of estrous cyclicity in aging C57BL/6J mice: I. Cycle frequency, length and vaginal cytology. Biol Reprod 1982; 27: 327–339. [DOI] [PubMed] [Google Scholar]
- Chen B, Pan H, Zhu L, Deng Y, Pollard JW.Progesterone inhibits the estrogen-induced phosphoinositide 3-kinase→AKT→GSK-3beta→cyclin D1→pRB pathway to block uterine epithelial cell proliferation. Mol Endocrinol 2005; 19: 1978–1990. [DOI] [PubMed] [Google Scholar]
- Tong W, Pollard JW.Progesterone inhibits estrogen-induced cyclin D1 and cdk4 nuclear translocation, cyclin E- and cyclin A-cdk2 kinase activation, and cell proliferation in uterine epithelial cells in mice. Mol Cell Biol 1999; 19: 2251–2264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Christenson LK, Nothnick WB.Regulation of MMP-9 expression and activity in the mouse uterus by estrogen. Mol Reprod Dev 2007; 74: 321–331. [DOI] [PubMed] [Google Scholar]
- Zhang X, Hoang E, Nothnick WB.Estrogen-induced uterine abnormalities in TIMP-1 deficient mice are associated with elevated plasmin activity and reduced expression of the novel uterine plasmin protease inhibitor serpinb7. Mol Reprod Dev 2009; 76: 160–172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu H, Wang D, Malarkey WB.A PCR-derived, nonisotopic-labeled prolactin cRNA probe suitable for in situ hybridization. Endocr Res 1995; 21: 793–802. [DOI] [PubMed] [Google Scholar]
- Nothnick WB.Disruption of the tissue inhibitor of metalloproteinase-1 gene results in altered reproductive cyclicity and uterine morphology in reproductive-age female mice. Biol Reprod 2000; 63: 905–912. [DOI] [PubMed] [Google Scholar]
- Wood GA, Fata JE, Watson KL, Khokha R.Circulating hormones and estrous stage predict cellular and stromal remodeling in murine uterus. Reproduction 2007; 133: 1035–1044. [DOI] [PubMed] [Google Scholar]
- Martin TA, Watkins G, Mansel RE, Jiang WG.Hepatocyte growth factor disrupts tight junctions in human breast cancer cells. Cell Biol Int 2004; 28: 361–371. [DOI] [PubMed] [Google Scholar]
- Satterfield MC, Dunlap KA, Hayashi K, Burghardt RC, Spencer TE, Bazer FW.Tight and adherens junctions in the ovine uterus: differential regulation by pregnancy and progesterone. Endocrinology 2007; 148: 3922–3931. [DOI] [PubMed] [Google Scholar]
- Bagchi IC, Li Q, Cheon YP, Mantena SR, Kannan A, Bagchi MK.Use of the progesterone receptor antagonist RU 486 to identify novel progesterone receptor-regulated pathways in implantation. Semin Reprod Med 2005; 23: 38–45. [DOI] [PubMed] [Google Scholar]
- Finn CA, Martin L.Endocrine control of gland proliferation in the mouse uterus. Biol Reprod 1973; 585–588. [DOI] [PubMed]
- Tibbetts TA, Mendoza-Meneses M, O'Malley BW, Conneely OM.Mutual and intercompartmental regulation of estrogen receptor and progesterone receptor expression in the mouse uterus. Biol Reprod 1998; 59: 1143–1152. [DOI] [PubMed] [Google Scholar]
- Wilson SE, Chen L, Mohan RR, Liang Q, Liu J.Expression of HGF, KGF, EGF and receptor messenger RNAs following corneal epithelial wounding. Exp Eye Res 1999; 68: 377–397. [DOI] [PubMed] [Google Scholar]
- Amano O, Matsumoto K, Nakamura T, Iseki S.Expression and localization of hepatocyte growth factor in rat submandibular gland. Growth Factors 1994; 10: 145–151. [DOI] [PubMed] [Google Scholar]
- Wang Y, Selden C, Farnaud S, Calnan D, Hodgson HJ.Hepatocyte growth factor (HGF/SF) is expressed in human epithelial cells during embryonic development: studies by in situ hybridization and Northern blot analysis. J Anat 1994; 185: 543–551. [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Selden AC, Morgan N, Stamp GW, Hodgson HJ.Hepatocyte growth factor/scatter factor expression in human mammary epithelium. Am J Pathol 1994; 144: 675–682. [PMC free article] [PubMed] [Google Scholar]
- Parrott JA, Skinner MK.Expression and action of hepatocyte growth factor in human and bovine normal ovarian surface epithelium and ovarian cancer. Biol Reprod 2000; 62: 491–500. [DOI] [PubMed] [Google Scholar]
- Uzumcu M, Pan Z, Chu Y, Kuhn PE, Zachow R.Immunolocalization of the hepatocyte growth factor (HGF) system in the rat ovary and the anti-apoptotic effect of HGF in rat ovarian granulosa cells in vitro. Reproduction 2006; 132: 291–299. [DOI] [PubMed] [Google Scholar]
- Finlay TH, Katz J, Kirsch L, Levitz M, Nathoo SA, Seiler S.Estrogen-stimulated uptake of plasminogen by the mouse uterus. Endocrinology 1983; 112: 856–861. [DOI] [PubMed] [Google Scholar]
- Henrikson KP, Hall ES, Lin Y.Cellular localization of tissue factor and prothrombin in the estrogen-treated immature rat uterus. Biol Reprod 1994; 50: 1145–1150. [DOI] [PubMed] [Google Scholar]