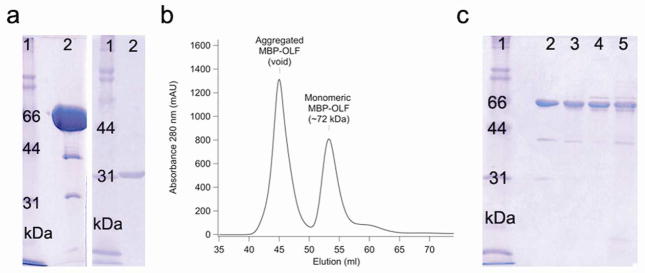
Figure 1.
Protein purification. a) SDS-PAGE analysis of purified MBP-OLF(wt) and cleaved MYOC-OLF. Left: lane 2, purified, concentrated (monomeric) MBP-OLF(wt) (72 kDa, ~ 10 mg ml−1). Right: lane 2, cleaved MYOC-OLF (~31 kDa, ~ 0.5 mg ml−1) by SDS-PAGE. b) SEC chromatograph of MBP-OLF(wt) displaying aggregated MBP-OLF and monomeric MBP-OLF. c) SDS-PAGE analysis of purified MBP-OLF mutants. Lane 2, MBP-OLF(D380A); lane 3, MBP-OLF(I477S); lane 4, MBP-OLF(I477N); lane 5, MBP-OLF(K423E). Low levels of MBP (~ 40 kDa) do not impede thermal stability measurements (see text). Gels include molecular mass standards as lane 1.