Abstract
Aging is associated with increased susceptibility to hypoxic/ischemic insult and declines in behavioral function which may be due to attenuated adaptive/defense responses. We investigated if diet-induced ketosis would improve behavioral performance in the aged rats. Fischer 344 rats (3- and 22-month-old) were fed standard (STD) or ketogenic (KG) diet for 3 weeks and then exposed to hypobaric hypoxia. Cognitive function was measured using the T-maze and object recognition tests. Motor function was measured using the inclined-screen test. Results showed that KG diet significantly increased blood ketone levels in both young and old rats. In the aged rats, the KG diet improved cognitive performance under normoxic and hypoxic conditions; while motor performance remained unchanged. Capillary density and HIF-1α levels were elevated in the aged ketotic group independent of hypoxic challenge. These data suggest that diet-induced ketosis may be beneficial in the treatment of neurodegenerative conditions.
1 Introduction
The brain, unlike other organs, is normally completely dependent on glucose, but is capable of using ketones as an alternate energy source, as occurs with prolonged starvation or chronic feeding of a high fat-low carbohydrate (ketogenic, KG) diet. The aging population is subject to increased rates of morbidity and mortality as a result of ischemic and hypoxic events related to stroke and neurodegenerative disease. There are many physiological factors that play a role in recovery and survival. These include those related to stabilization of energy metabolism, the ability to defend against oxidative stress and maintain vascular integrity and density [1]. We have previously shown that diet-induced ketosis increased cortical capillary density [2] and reduced CMRglu in the young rats [3], and was neuroprotective against focal ischemic insult [4]. One mechanism related to ketotic-induced neuroprotection may be through the upregulation of hypoxic inducible factor-1alpha (HIF-1α) [4, 5]. Aging is associated with increased susceptibility to ischemic/hypoxic insult [6] and cognitive decline [7]. In this study, we investigated if diet-induced ketosis improves behavioral performance in the aged rats. Capillary density and HIF-1αresponses were determined in the young and aged rats fed a STD or KG diet under normoxic and hypoxic conditions.
2 Methods and Materials
2.1 Animal Preparation and Hypoxic Exposure
Male Fischer 344 rats (young: 3-month-old, and aged: 22-month-old) were purchased and allowed to acclimatize in the animal facility at Case Western Reserve University for 1 week before being studied. Rats were fasted overnight before being assigned to two groups fed with either STD or KG diets [2] for 3 weeks. Body weights and ketotic markers such as plasma levels of BHB were measured twice a week. Hypoxic rats were kept in hypobaric chambers for up to 3 weeks at a constant pressure of 0.5 ATM (380 mmHg, equivalent to 10% normobaric oxygen) except for a maximum of 3 h daily when the pressure is returned to atmospheric for cage cleaning, water, food and weighing and behavioral tests. The normoxic control rats were housed in the same room to ensure identical ambient conditions [8].
2.2 Behavioral Tests
(1) Object recognition test: This test is based on the natural tendency of rodents to investigate a novel object instead of a familiar one as well as their innate tendency to re-start exploring when they were presented with a novel environment. The number of times and length of time inspecting the objects over the different trials was calculated. (2) T-maze test: This test is used for general cognitive function and it is based on the innate preference of animals to explore an arm that has not been previously explored (spontaneous alternations). The number of arms entered as well as the sequence of entries was recorded and the alternation rate was calculated. (3) Inclined screen test (60 and 90°): This is to test balance, muscle strength and coordination. Latency to climb to the top of the screen and/or latency to fall was recorded, and overall score was calculated.
2.3 Immunoreactivity of HIF-1α and GlUT-1
HIF-1α protein content was analyzed in cortical tissue lysates by Western blot analysis [2]. Capillary density (number/mm2) in cortical regions was assessed by immunohistochemical staining with GLUT-1 [2, 9].
2.4 Statistical Analysis
Quantitative data are expressed as mean ± SD. Statistical analyses were performed using SPSS V13.0 for windows. The comparison between any two groups was analyzed with a t-test. Significance was considered at the level of p < 0.05.
3 Results
3.1 Physiological Variables
During the 3-week diet period, the body weight gain was 12–20% in the aged and young, respectively. Hematocrits were similar between the two diet groups (47 ± 2, n = 6) in the young. However, hematocrit was elevated in the KG-diet aged group (57 ± 4, n = 12) prior to hypoxic exposure. After 3-week hypoxia, hematocrit (%) reached about 70 in both diet groups young and old. Ketosis as measured by blood ketone levels (mM) ranged 0.8–2.8 following 3 weeks of KG diet in young and old and remained unchanged during hypoxic exposure.
3.2 Behavioral Performance
Cognitive function was assesses by a T-maze test and an object recognition test. In the T-maze test, a high alternation rate and quick decision on choosing which arm to enter are indicatives of sustained cognition as the animals must remember which arm was entered last to not re-enter it. In the young rats, the STD and KG rats had similar alternation rate (~53%) and time on choosing arms (~ 30 s). Compared to the young rats, the STD-diet aged rats had lower alternation rate and longer time to choose arms; however, the KG-diet aged group had similar performance compared to the young. In the aged rat, the KG-diet group had a significantly higher alternation rate (%, 63 ± 21 vs. 37 ± 21, n = 7 each) and significantly shorter time on choosing arms (seconds, 28 ± 15 vs. 48 ± 7, n = 7 each) than that of the STD-diet group. In the object recognition test, the percent of new object exploration is higher with good cognitive function. Under normoxic conditions, on either diet, the younger rats had significantly higher new object exploration (Fig. 1) compared to the aged rats. Yet, in the aged rats, the new object exploration was significantly increased in the KG-diet group compared to the STD-diet group under normoxic condition (Fig. 1) and at 1 day hypoxia (%, 80 ± 17 vs. 55 ± 29, n = 7 each).
Fig. 1.
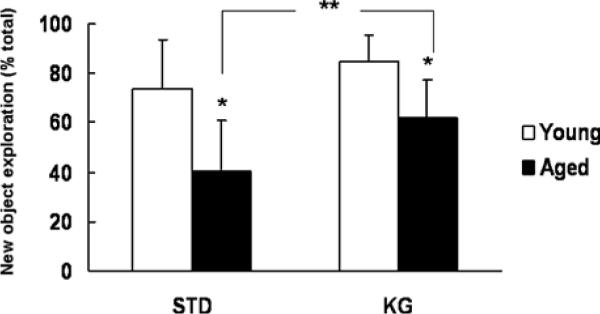
Object recognition test. New object exploration (%)=(exploration time on new object/total exploration time) × 100. Values are mean± SD, in the young, STD: n=4, KG: n=3; in the aged, n=7 each. * indicates significant difference compared to the young with same diet (t-test, p< 0.05); ** indicates significant difference between STD and KG diet groups (t-test, p < 0.05)
The motor function was assessed by an inclined screen test (60 and 90°). Latency to climb to the top and/or latency to fall was recorded, and overall score was calculated using a scoring system as following: 2=climb to the top; 1=fail to climb to the top but stay on the screen; 0=fall. Under normoxic conditions, the aged rats had significantly lower scores (2.0 ± 1.4 and 2.6 ± 1.8, STD and KG groups respectively, n=11 each) for 60-degree incline compared to the young rats with the same diet (4.5 ± 1.3 and 5.0 ± 1.0, STD and KG groups, respectively, n=4 each). Similar trends were found in the 90-degree incline test, with lower scores in each group. There was no significant difference between the KG-diet group and the STD diet group in any age group under normoxic or hypoxic conditions.
3.3 Detection of HIF-1α Protein in Brain Cortex
Levels of HIF-1α in brain cortex were detected using Western blot analysis (Fig. 2). The data show an upregulation of HIF-1with ketosis in the young and old brain under normoxic conditions (STD vs. KG), similar to what we have previously reported in the young adult [4].
Fig. 2.
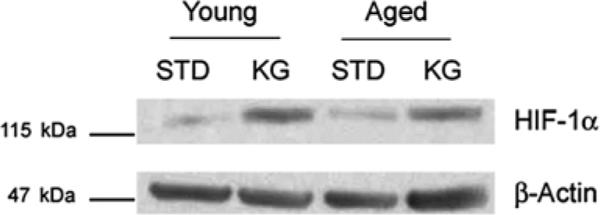
Western blot analysis showing HIF-1α expression in STD and KG rat brain cortex in both young and aged groups
3.4 Capillary Density: GLUT-1 Immunoreactivity
Increase in capillary counts as measured by GLUT-1 immunoreactivity in coronal-cortical sections of aged rat brain after 3 weeks of feeding diets (STD and KG diets. Following hypoxic exposure, capillary density increased 40% in the aged and 60% in the young fed STD diet compared to normoxia. In the KG-diet aged group the capillary density increased similar to what was observed with hypoxic exposure. There was no additive effect of hypoxia with KG diet.
4 Discussion
The key findings derived from this study are that there are quantifiable declines in cognitive and motor skills with aging in the Fisher 344 rat model, and that at least the cognitive deficits can be overcome through dietary intervention. These studies also show that a KG diet can be tolerated in both young and aged Fischer rats, consistent with what we have previously reported [2]. Moderate ketosis was observed following 3-wk feeding of the KG diet in both age groups. Cognitive function was improved with ketosis in the aged rat under normoxic conditions and when challenged by environmental hypoxia. The data also show that brain capillary density was lower and its responsiveness to hypoxia was attenuated compared to young rats. In the aged rats, ketosis induced angiogenesis and increased capillary density equivalent to that observed after hypoxic exposure in young rats. We speculate that the KG diet revitalized the neurovascular unit in the older rats helping to support cognitive function. Thus, diet-induced ketosis may improve adaptation process to hypoxia through elevated HIF-1α and increased capillary density.
References
- 1.Prins ML. Cerebral metabolic adaptation and ketone metabolism after brain injury. J Cereb Blood Flow Metab. 2008;28(1):1–16. doi: 10.1038/sj.jcbfm.9600543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Puchowicz MA, Xu K, Sun X, Ivy A, Emancipator D, LaManna JC. Diet-induced ketosis increases capillary density without altered blood flow in rat brain. Am J Physiol Endocrinol Metab. 2007;292(6):E1607–E1615. doi: 10.1152/ajpendo.00512.2006. [DOI] [PubMed] [Google Scholar]
- 3.Koppaka SS, Puchowicz MA, LaManna JC, Gatica JE. Effect of alternate energy substrates on mammalian brain metabolism during ischemic events. Adv Exp Med Biol. 2008;614:361–370. doi: 10.1007/978-0-387-74911-2_40. [DOI] [PubMed] [Google Scholar]
- 4.Puchowicz MA, Zechel JL, Valerio J, Emancipator DS, Xu K, Pundik S, LaManna JC, Lust WD. Neuroprotection in diet-induced ketotic rat brain after focal ischemia. J Cereb Blood Flow Metab. 2008;28(20):1907–1916. doi: 10.1038/jcbfm.2008.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chavez JC, LaManna JC. Activation of hypoxia-inducible factor-1 in the rat cerebral cortex after transient global ischemia: potential role of insulin-like growth factor-1. J Neurosci. 2002;22(20):8922–8931. doi: 10.1523/JNEUROSCI.22-20-08922.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Xu K, Sun X, Puchowicz MA, LaManna JC. Increased sensitivity to transient global ischemia in aging rat brain. Adv Exp Med Biol. 2007;599:199–206. doi: 10.1007/978-0-387-71764-7_26. [DOI] [PubMed] [Google Scholar]
- 7.Schulz D, Kouri C, Huston JP. Behavior on the water maze platform: relationship to learning and open field exploration in aged and adult rats. Br Res Bull. 2007;74(4):206–215. doi: 10.1016/j.brainresbull.2007.06.010. [DOI] [PubMed] [Google Scholar]
- 8.Xu K, Puchowicz MA, LaManna JC. Renormalization of regional brain blood flow during prolonged mild hypoxic exposure in rats. Br Res. 2004;1027(1–2):188–191. doi: 10.1016/j.brainres.2004.08.046. [DOI] [PubMed] [Google Scholar]
- 9.Pichiule P, LaManna JC. Angiopoietin-2 and rat brain capillary remodeling during adaptation and deadaptation to prolonged mild hypoxia. J Appl Physiol. 2002;93(3):1131–1139. doi: 10.1152/japplphysiol.00318.2002. [DOI] [PubMed] [Google Scholar]


