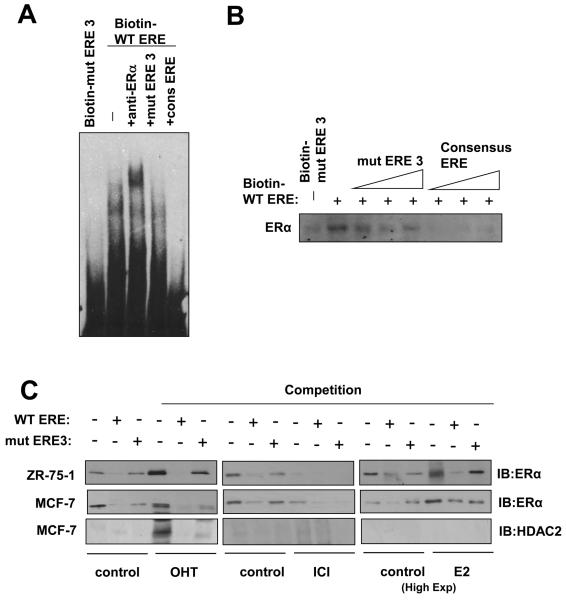
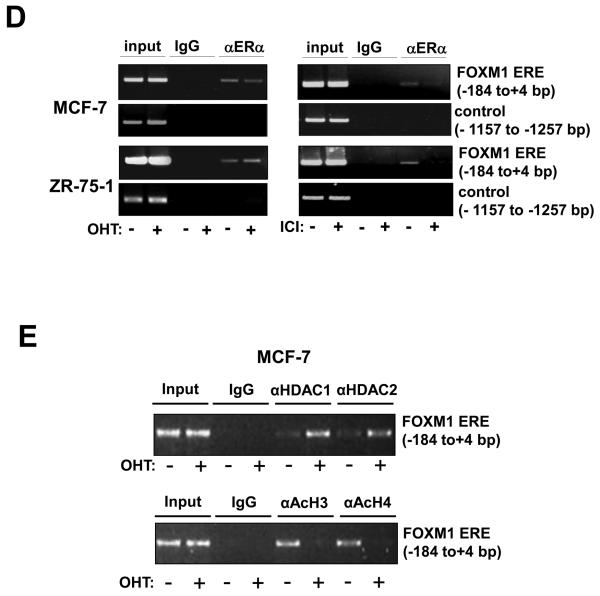
Figure 3. ERα binds directly to the ERE on the FOXM1 promoter.
A) Biotinylated wild-type ERE or the mutant ERE3 oligonucleotides were incubated with MCF-7 cell lysates in the presence or absence of 10x molar excess of non-biotinylated ERE3 or consensus ERE oligonucleotides or an anti-ERα antibody. In brief, gel shift assays (20μl total volume) contained 3μg nuclear extracts and 50ng/μl poly (dI-dC) in 1x binding buffer and 20pmol unlabelled oligonucleotides. For the supershifts, 1μl of the anti-ERα (Santa Cruz; H222) antibody were included in the reaction mix. The mixtures were pre-incubated at room temperature for 15 min, followed by addition of 100fmol of biotin-labelled double stranded estrogen response element (ERE-conc. 5′-GCCGATTGGCGACGTTCGGTCACGCTGACCTTAACGCTCCGCCGGCG-3′ 5′-CGCCGGCGGAGCGTTAAGGTCACGCTGACCGAACGTCGCCAATCGGC-3′), (ERE-wt 5′-GCCGATTGGCGACGTTCCGTCACGTGACCTTAACGCTCCGCCGGCG-3′, 5′-CGCCGGCGGAGCGTTAAGGTCACGTGACGGAACGTCGCCAATCGGC-3′), or (mERE3 5′-GCCGATTGGCGACGTTCCGTAACGTTACGTTAACGCTCCGCCGGC-3′, 5′-CGCCGGCGGAGCGTTAACGTAACGTTACGGAACGTCGCCAATCGGC-3′), and incubation at room temperature for 20 min, followed by a 30 min incubation on ice. Protein-DNA complexes were separated on 5% 0.5 × TBE polyacrylamide gels. The electrophoretic transfer of the binding reactions to the nylon membrane, and the detection of the biotin labelled DNA by chemiluminescence, were performed according to the PIERCE kit's protocols. B) Nuclear extracts from MCF-7 cells were incubated with biotinylated oligonucleotides representing region of the FOXM1 promoter containing the ERE or the mutated ERE3 site in the absence or presence of molar excess of non-biotinylated ERE3 or consensus ERE oligonucleotides. Proteins binding to the biotinylated oligonucleotides were pulled-down using streptavidine agarose beads and analysed by western blot using specific antibodies as indicated. C) The nuclear extracts (Fig. S1) from MCF-7 and ZR-75-1 cells with or without OHT or ICI for 24 h were also examined by pull-down assays using biotinylated wild-type or mutant (mERE3) oligonucleotides as described above. D) Chromatin immunoprecipitation (ChIP) analysis of the human FOXM1 promoter. MCF-7 and ZR-75-1 cells untreated or treated with ICI or OHT for 24 h were used for ChIP assays using IgG, anti-ERα antibodies as indicated. After crosslink reversal, the co-immunoprecipitated DNA was amplified by PCR using primers amplifying the FOXM1 ERE containing region (−184/+4) and a control region (−1157/−1257), and resolved in 2% agarose gel. E) MCF-7 untreated or treated with OHT for 24 h were used for ChIP assays using IgG, antibodies against acetylated H3 and H4, HDAC1 and HDAC2 as described above. Representative data from three independent experiments are shown.