Figure 1.
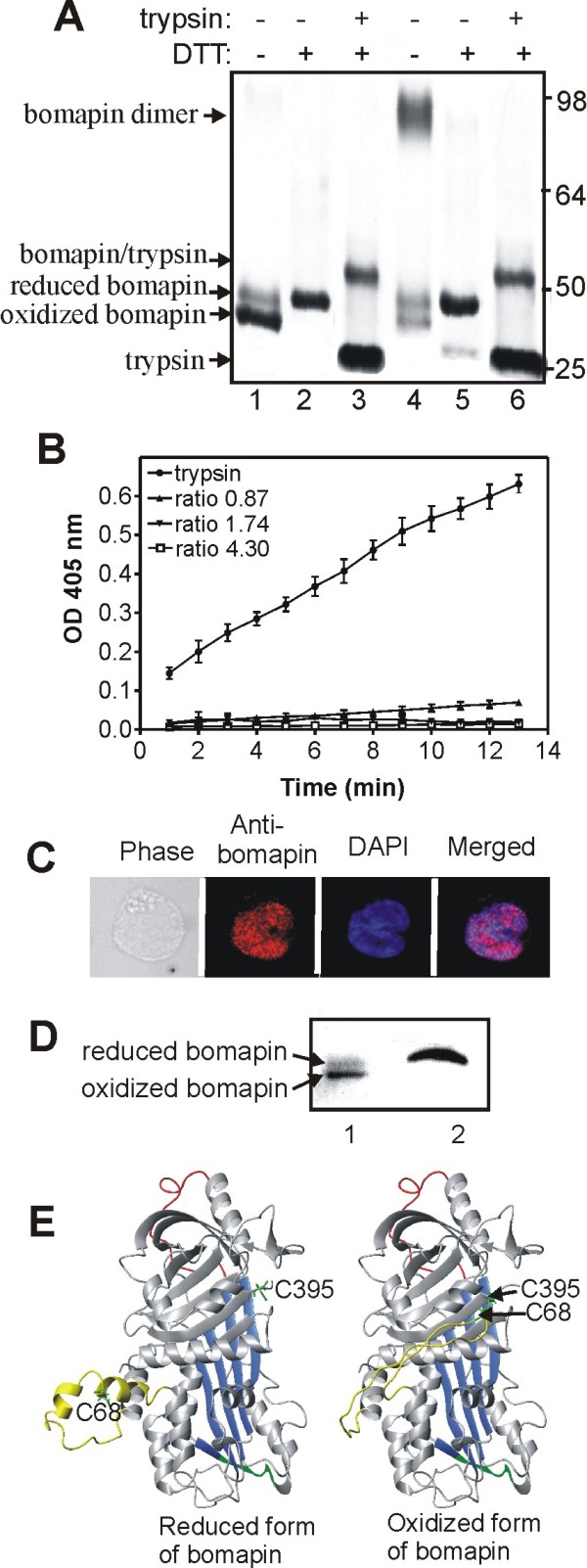
Conformation of the recombinant and naturally expressed bomapin is redox-dependent. (A) Purified recombinant bomapin analyzed by SDS-PAGE followed by Coomassie blue staining: lanes 1 and 4, non-reduced bomapin eluted from ion-exchange column in peak 1 and peak 2 (respectively); lanes 2 and 5, DTT-reduced bomapin from peak 1 and peak 2 (respectively); lanes 3 and 6, non-reduced bomapin from peak 1 and peak 2 (respectively) incubated with a 4-fold molar excess of trypsin and then analyzed under reducing conditions. (B) Indirect chromogenic assay to detect inhibitory activity of bomapin. Trypsin alone or trypsin pre-incubated with different amounts of bomapin (at bomapin/trypsin molar ratios as indicated) was mixed with S-2288 chromogenic substrate, and residual trypsin activity was measured at 405 nm in 1 min time intervals. (C) Immuno-localization of naturally expressed bomapin in THP1 cells. The cells were stained with rabbit anti-bomapin antibodies followed by Alexa-Fluor 568-labeled secondary antibody; DNA was stained with DAPI. (D) Bomapin was immunoprecipitated from HL60 cell extract and analyzed by SDS-PAGE followed by western blot: lane 1, non-reduced bomapin; lane 2, DTT-reduced bomapin. (E) In silico homology models of bomapin in its reduced and oxidized forms. The central β-sheet A is shown in blue, the reactive centre loop in red, the CD-loop in yellow, and the two cysteine residues in green.
