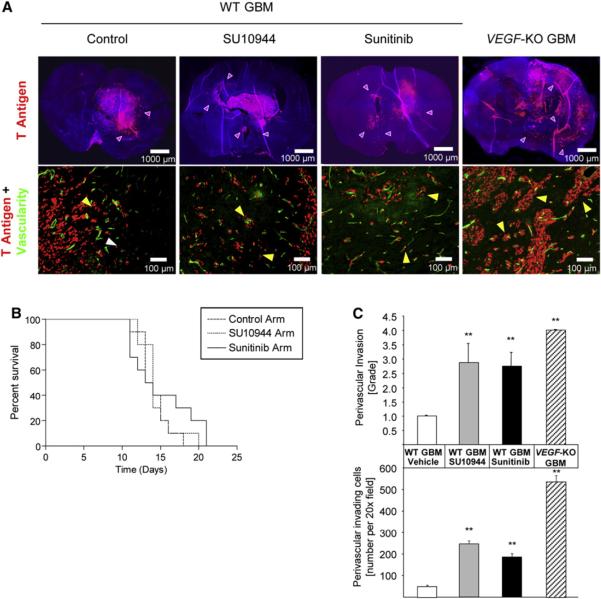
Figure 6. Effects of VEGFR-Selective Kinase Inhibitors on an Orthotopic Mouse Model of Glioblastoma Multiforme.
(A) Top row: immunohistological analysis of glioblastoma multiforme (GBM) with fluorescent SV40 T antigen staining (red) on whole brain sections counterstained with DAPI (blue). Control wild-type (WT) GBMs appear less invasive than WT GBMs treated with SU10944 or sunitinib, or VEGF-KO GBMs (purple arrowheads). Bottom row: high-magnification images of fluorescent detection of tumor cells (anti-T antigen antibody; red) and the perfused vasculature (FITC-lectin, green). WT GBM cells infiltrate as single cells into the brain parenchyma without associating with blood vessels (white arrowheads) or invade alongside blood vessels in the brain (perivascular invasion; yellow arrowheads). GBMs treated with either SU10944 or sunitinib as well as VEGF-KO GBMs are more invasive and predominantly migrate along blood vessels (yellow arrowheads).
(B) Kaplan-Meier survival curves of WT GBM-bearing mice untreated or treated with a relatively selective VEGFR inhibitor (SU10944) or a multitargeted VEGFR inhibitor (sunitinib) starting 3 days after inoculation. The effects on survival observed with SU10944 and sunitinib treatment were minimal or modest, respectively, and were not statistically significant.
(C) Top: grading of perivascular invasion was performed on a scale from 1 to 4, where 1 indicates minimal distant spread of tumor cells and 4 indicates substantial and marked distant spread; this parameter indicated a significant increase in perivascular invasion in response to both pharmacological treatments and in the VEGF-KO GBMs (**p < 0.01). Bottom: quantification of perivascular invasive mode by immunohistochemical analysis on tumor sections. Perivascular invasive cells, counted as the number of cells in the tumor periphery tightly associated with vessels, were significantly increased by both antiangiogenic small-molecule treatments and the genetic ablation of VEGF-KO GBM cells (**p < 0.01). Error bars indicate ± SD.