Figure 3.
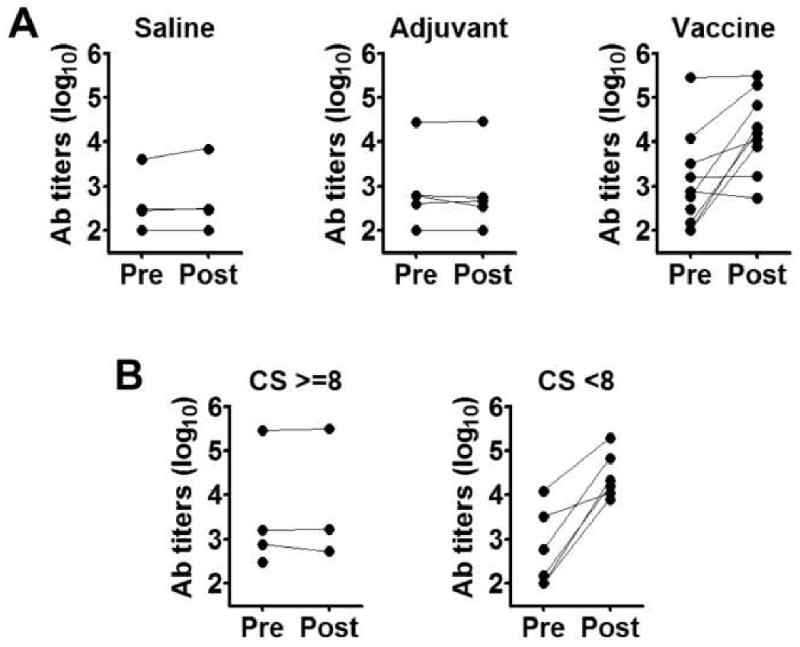
Leish-111f antibody titers in the three Blinded trial treatment groups and the relationship between disease severity and Leish-111f responsiveness within the Vaccine group. (A) Plasma samples were collected at pre- and post-vaccination time points from dogs in the Saline, Adjuvant, and Vaccine groups, and evaluated for antibody titers to Leish-111f by ELISA. Reciprocal endpoint titers for pre- and post-vaccination samples (filled circles) are shown for each dog; one dog in the Vaccine group died before a post-vaccination sample could be obtained. (B) ELISA titers of Vaccine-group dogs were re-plotted in the categories CS ≥8 and CS <8 based on CS at Day 0.
