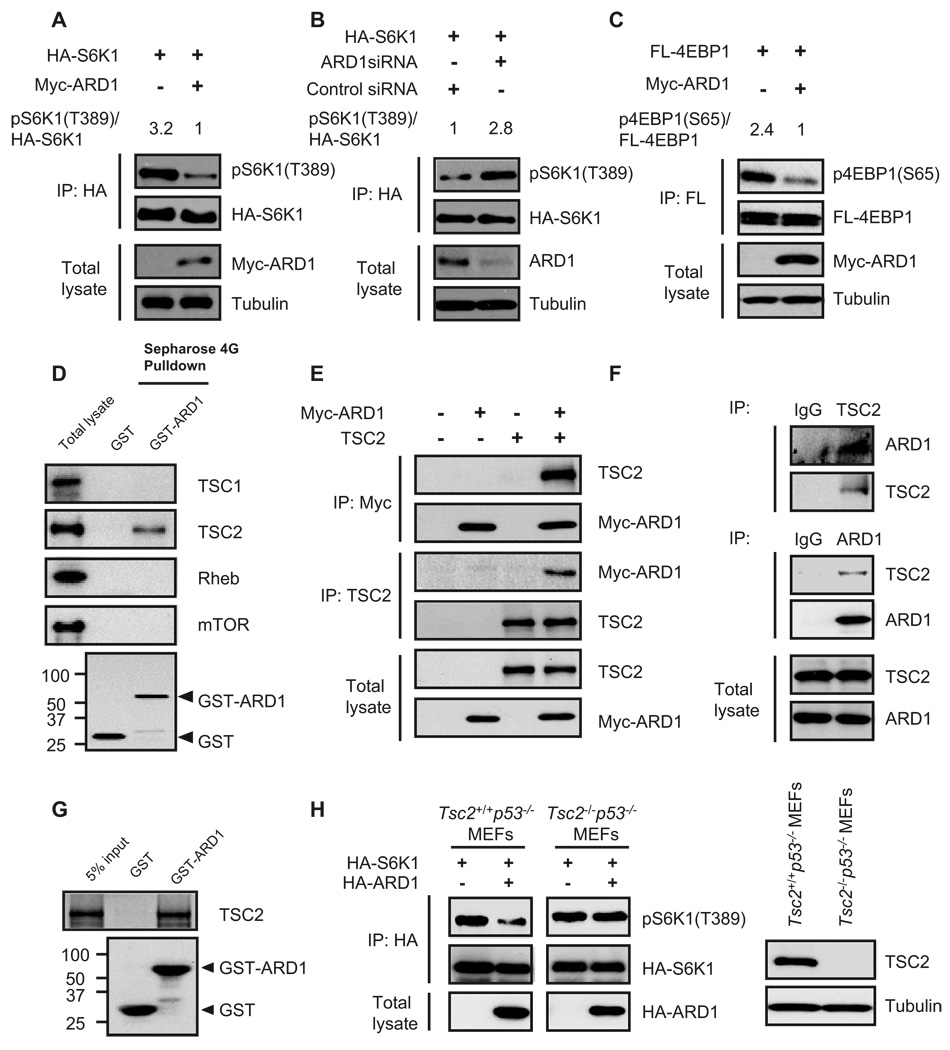
Fig. 4.
ARD1 inhibition of mTOR activity through TSC2. (A) Myc-ARD1 decreased S6K1 phosphorylation [pS6K1(T389)] in HEK293T cells. (B) ARD1 knockdown with siRNAs increased S6K1 phosphorylation [pS6K1(T389)] in HEK293T cells. (C) Myc-ARD1 decreased 4EBP1 phosphorylation [p4EBP1(S65)] in HEK293T cells. (D) TSC2, but not TSC1, Rheb, or mTOR, associated with GST-ARD1. GST or GST-ARD1 protein was pulled down with Sepharose 4G beads, and the associated proteins were analyzed by immunoblotting. (E) Interactions between exogenous ARD1 and TSC2 proteins. Lysates of HEK293T cells cotransfected with Myc-ARD1 and TSC2 were analyzed with antibodies directed against the Myc tag and TSC2 by reciprocal coimmunoprecipitation and immunoblotting. (F) Interaction between endogenous ARD1 and TSC2 proteins. Lysates of MDA-MB-435 cells were analyzed with antibodies directed against ARD1 and TSC2 by reciprocal coimmunoprecipitation and immunoblotting. (G) ARD1 interacted directly with TSC2. In vitro transcribed and translated TSC2 proteins were incubated with GST or GST-ARD1 proteins and then pulled down with Sepharose 4G beads. (H) Transient transfection of HA-ARD1 decreased pS6K1(T389) in Tsc2+/+ p53−/− MEFs but not in Tsc2−/− p53−/− MEFs.