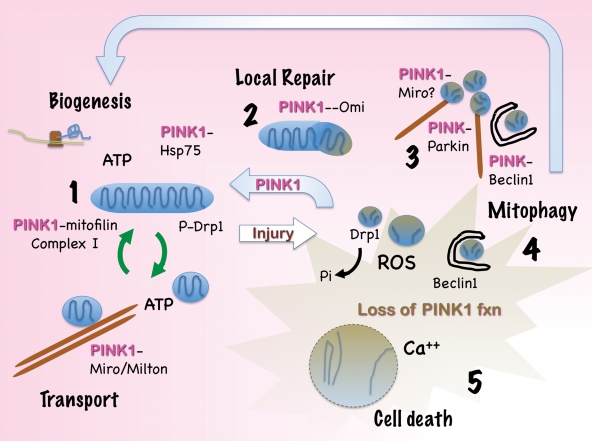
Figure 3.
A pivotal role for PINK1 in mitochondrial quality control. Emerging data indicate that PINK1 acts at multiple levels to promote mitochondrial health. (1) PINK1 deficiency studies suggest that endogenous PINK1 serves to maintain mitochondrial complex I activity, potentially through stabilization of cristae structures and electron transport chain components through interactions with mitofilin or the chaperone Hsp75/TRAP1. PINK1 also suppresses basal and 6-hydroxydopamine-induced mitochondrial ROS production and regulates mitochondrial calcium homeostasis. Interactions involving Miro/Milton suggest a role in regulating microtubule-dependent mitochondrial transport. (2) Maintenance of mitochondrial integrity may also involve interactions with chaperones or indirect regulation of the mitochondrial protease Omi/HTrA2 to effect localized repair. (3) With severe mitochondrial injury, Parkin is recruited to mitochondria, resulting in mitochondrial ubiquitination and formation of large perinuclear aggregates with features of aggresomes. These are cleared by Beclin 1-dependent autophagy, and expression of full-length PINK1 facilitates Parkin translocation, mitochondrial aggregation and eventual clearance. Interactions of PINK1 with Parkin, Beclin 1 and potentially Miro/Milton may contribute to this clearance. (4) Under conditions of PINK1 deficiency, the earlier tiers of mitochondrial quality control are compromised. Increased ROS and calcium signals trigger dephosphorylation of Drp1, mitochondrial fission and mitophagy, which plays a neuroprotective role in this setting. Interestingly, Parkin and Beclin 1 also function to clear PINK1-deficient mitochondria, suggesting that autophagy functions as one of the final lines of defense against mitochondrial injury. (5) Dysregulation of autophagy involving either insufficient, or excessive mitochondrial clearance, contributes to neuronal cell death.