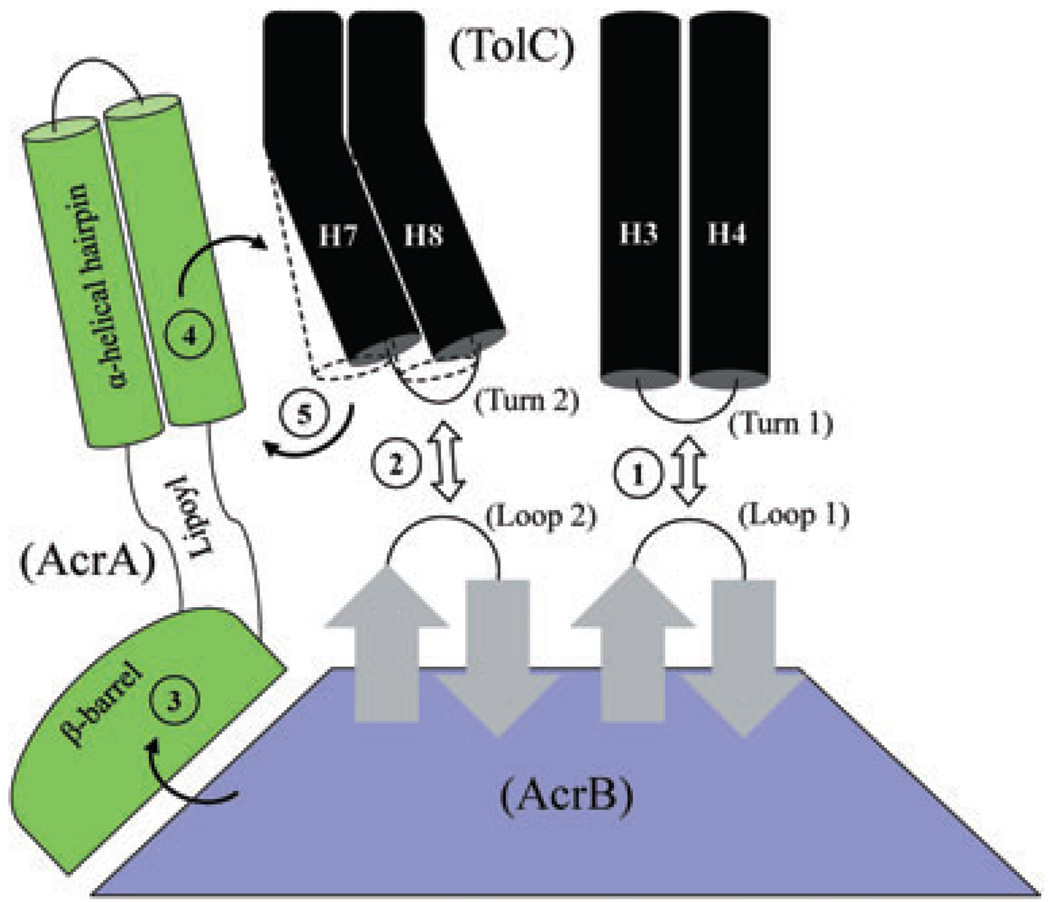
Fig. 10.
A cartoon depicting possible stepwise interactions between TolC, AcrA and AcrB, leading to full dilation of the TolC aperture/channel. Static (H3/H4) and mobile (H7/H8) paired helices guarding the TolC aperture are shown as black cylinders. In the resting stage, the TolC aperture is kept closed by intra- and inter-subunit ionic bridges. The three domains of AcrA and only the top half of AcrB, including its TolC-docking domain, are shown. It is envisaged that initial interactions between AcrB hairpin loops and TolC turns (steps 1 and 2) can trigger partial opening of the TolC aperture; however, these bilateral interactions are not sufficient to fully dilate the TolC aperture. Binding of substrates and protons induce conformational changes in AcrB that are then transduced via AcrA’s β-barrel and lipoyl domains (step 3) to its TolC-proximal α-helical hairpin domain. Direct interactions between the conformationally induced α-helical hairpin domain of AcrA and intra-protomer grooves formed between H3/H4 and H7/H8 pairs (step 4) allow for outward extension of the TolC helices (outlined by dashed lines), leading to full dilation of the TolC aperture (step 5). The model is based on proposals by Fernández-Recio et al. (2004), Murakami et al. (2006), Seeger et al. (2006) and Bavro et al. (2008). The AcrA suppressors obtained in this study dilate the TolC aperture in an AcrB-dependent manner but without receiving its conformational energy generated by the drug/proton translocation cycle.