Abstract
In the rat, neonatal administration of testosterone propionate to a castrated male causes masculinization of behavior. However, if an intact male is treated neonatally with testosterone (hyper-androgen condition), male sexual behavior in adulthood is disrupted. There is a possibility that the hyper-androgen treatment is suppressing male sexual behavior by altering the male’s partner preference and thereby reducing his motivation to approach the female. If so, this would suggest that exposure to supra-physiological levels of androgen during development may result in the development of male-oriented partner preference in the male. To test this idea, male rats were treated either postnatally or prenatally with testosterone, and partner preference and sexual behavior were examined in adulthood. The principal finding of this study was that increased levels of testosterone during early postnatal life, but not prenatal, decreased male sexual behavior and increased the amount of time a male spent with a stimulus male, without affecting the amount of time spent with a stimulus female during partner preference tests. Thus, the reduction in male sexual behavior produced by early exposure to high levels of testosterone is not likely due to a reduction in the male’s motivation to approach a receptive female.
Keywords: partner preference behavior, sexual behavior, sexual orientation, testosterone, neonatal development, laboratory rats
INTRODUCTION
In male rats, as in many other mammalian species, exposure to endogenous testosterone during perinatal development causes masculinization, the enhancement of neural systems that mediate male-typical responses, and defeminization, the suppression of neural systems that mediate female-typical responses (Adkins-Regan, 1988; Bakker, 2003; Baum, 1979). These effects of testosterone during early development are often referred to as “organizational effects” and can occur both pre- and postnatally.
Prenatally, male rats show a testosterone surge that starts on gestation day 16 and lasts through gestation day 20 (Ward et al., 2003). This surge has been shown to be important for the differentiation of sexual behavior (Hoepfner and Ward, 1988). Male offspring of mothers treated with an anti-androgen during gestation show feminized behavior in adulthood (Neumann et al., 1966). Females that develop between two males in utero show more male-like sexual behavior in adulthood than females located between two females, and this effect is blocked by prenatal treatment with an anti-androgen (Clemens et al., 1978). Finally, females treated with testosterone during both the pre- and postnatal period show a greater increase in the expression of male-like sexual behavior as adults than after postnatal androgen treatment alone (Pollak and Sachs, 1975; Ward, 1969).
Castration at birth eliminates or greatly reduces the expression of male sexual behavior in adulthood (Beach and Holz, 1946). Normal masculine behavior is reinstated, however, if testosterone is replaced immediately following castration (Beach et al., 1969). In addition, female rats treated neonatally with testosterone show, in some cases, an increase in male typical responses (Mullins and Levine, 1968; Whalen and Edwards, 1967), and males treated neonatally with the anti-androgen, flutamide, show decreases in the expression of male sexual behavior (Hernandez-Tristan and Cerezo, 2000). These findings support the idea that both pre- and postnatal endogenous testosterone mediates behavioral sex differentiation in rats.
Although testosterone is necessary for normal development of male sexual behavior, exogenous perinatal testosterone treatment of an intact male results in disruption of normal male sexual behavior (Diamond et al., 1973; Piacsek and Hostetter, 1984; Pollak and Sachs, 1975; Zadina et al., 1979). One factor that could mediate this effect of testosterone exposure is the possibility that the hyper-androgen treatment is reducing the male’s motivation to approach the female. Support for this idea can be found in both the animal and human literature. Although not statistically significant, male ferrets treated with testosterone perinatally appear less likely to approach a stimulus female than a stimulus male compared to control males (Baum et al., 1990). Prenatal hyper-androgen exposure has also been suggested to play a role in sexual orientation in humans. Some homosexual men have been shown to have larger genitalia (Bogaert and Hershberger, 1999), more masculine auditory evoked potentials (McFadden and Champlin, 2000), and more masculine 2D:4D digit length ratios (Rahman, 2005; Rahman and Wilson, 2003; Robinson and Manning, 2000) than heterosexual men (but see Berenbaum et al., 2009). All of these measures appear to be androgen-dependent during development, indicating that some homosexual men may be exposed to a higher level of androgen during development compared to heterosexual men.
Whereas several studies (Diamond et al., 1973; Piacsek and Hostetter, 1984; Pollak and Sachs, 1975; Zadina et al., 1979) have focused on the consummatory aspects of male sexual behavior after early exposure to elevated levels of testosterone, the current study expands these findings to include appetitive aspects of male sexual behavior including measures of partner preference. A male’s motivation to approach a partner can be measured in a preference test by examining the amount of time spent with each stimulus animal. We hypothesize that the reduction in male sexual behavior after early testosterone treatment is due, at least in part, to a decrease in the male’s attraction to the female. In the present study, the effect of exogenous testosterone on adult behavior was tested by treating intact male rats during either early postnatal development or prenatally with testosterone propionate and examining their adult partner preference and sexual behavior. The effects of early postnatal exposure to testosterone on serum testosterone and estradiol levels were also evaluated.
METHODS
Overview of Experiments
Three experiments were conducted in this study. In Experiment 1, the experimental males were exposed to early postnatal hormone treatments and stimulus males were untreated. In Experiment 2, experimental males were exposed to early postnatal hormone treatments and stimulus males were treated with the aromatase inhibitor 1,4,6 androstatriene-3,17-dione (ATD) during the early postnatal period as explained below. In Experiment 3, experimental males were exposed to prenatal hormone treatments and stimulus males were untreated.
Animals
Experimental Males (Experiments 1-3)
Time-mated pregnant Long-Evans rats (Charles River, Raleigh, NC) were housed individually in plastic cages (45.5 × 24 × 21 cm) with ad lib food and water (Experiment 1: TP n=10, C n=7; Experiment 2: TP n=10, C n=11; Experiment 3: TP n=5, Oil n=7). The dams were kept in a 14:10-hr light dark cycle with lights on at 01:00. Thirty one-inch paper towel strips were given to the dams for nest building material on gestation day (GD) 20. A subset of the male offspring of these dams became the experimental males of this study. Separate cohorts of pregnant dams were used for each experiment. In Experiments 1 and 2, on the day of birth (postnatal day [PND] 0), the litter was reduced to four male and four female pups to keep litter size uniform across groups. For litter reductions, the anogenital distance (AGD) for each pup was measured. The animals with the four shortest and the four longest measurements were retained to provide a mixed-gender litter since the AGD is shorter in females than in males. In Experiment 3, since prenatal testosterone increases AGD of both sexes, the seven animals with the largest AGD were kept to maximize the chance of picking males, and the animal with the smallest AGD was kept in an attempt to prevent forming an all-male litter. Thus, the experimental males used in the present study were not selected randomly at birth. The AGD is an indication of androgenic effects perinatally, and therefore the males used were selected on the basis of high androgen responsiveness.
Experimental Male Hormone Treatments (Experiments 1,2)
Silastic capsules (Dow Corning; inner diameter 1.47 mm; outer diameter 1.96 mm; length 5 mm) were used to administer either testosterone propionate (Sigma; TP) or cholesterol (Sigma; C) treatments starting on the day of birth. The pups were anesthetized using ice and the capsules were implanted subcutaneously (s.c.; one treatment per litter) through a small incision on the back of the animal. These animals are referred to as TP or C males, respectively. The incision was closed using superglue (Loctite). The pups were kept under a heat lamp for approximately one hour until the incision was dry. They were then placed back with their mothers. The implants were removed through an incision made near one end of the capsule on PND 21 under isoflurane anesthesia (Isoflo, Abbot Laboratories). First Aid Cream (Johnson and Johnson) was used on the incision, which was then closed with an Auto Clip (Clay Adams). The pups were weaned at this time and housed with same-sex littermates. Although the testosterone exposure is longer than other studies examining the effect of postnatal hyper-androgen exposure on behavior (Diamond et al., 1973; Piacsek and Hostetter, 1984; Pollak and Sachs, 1975; Zadina et al., 1979), previous studies from this laboratory show that treatment with steroid hormones or endocrine disrupting agents during this postnatal time period results in changes in the expression of adult behavior (Cummings et al., 2008; Henley et al., 2009). Two male pups randomly selected from each litter, and therefore, exposed to the same treatment, were used in these experiments. Behavioral tests began after the animals reached 90 days of age (Experiment 1: TP n=18, C n=14; Experiment 2: TP n=20, C n=21).
Experimental Male Hormone Treatments (Experiment 3)
Pregnant dams were treated with either TP in sesame oil (2 mg/0.1 ml/day) or oil vehicle on GD 16-20. Pups were weaned on PND 21 and housed with same-sex littermates until 90 days of age when behavioral tests were conducted. These males are referred to as TP and Oil males, respectively. Two male pups from each litter were randomly selected for this experiment, and behavioral tests began after the animals reached 90 days of age (TP n=9, C n=13).
Stimulus Females (Experiments 1-3)
Stimulus females were sexually experienced, gonadally intact, adult Long Evans female rats at least 60 days old (Charles River, Raleigh, NC). Prior to testing, a Silastic capsule containing 25% estradiol benzoate (Sigma) to cholesterol mixture was implanted s.c. on the back of the females while under isolurane anesthesia. The incision was closed with an Auto Clip and covered with First Aid Cream. After 4 weeks, the capsules were removed and reimplanted via a new incision in the neck. This was to prevent a loss of efficacy due to connective tissue growth around the capsule (personal observation). Stimulus females were injected s.c. with 0.5 mg progesterone four hours prior to partner preference and sexual behavior testing.
Stimulus Males (Experiments 1,3)
Sexually experienced, gonadally intact, adult Long Evans male rats at least 90 days old (Charles River, Raleigh, NC) were used as stimulus animals for the behavioral tests.
Stimulus Males (Experiment 2)
The stimulus males in Experiment 2 received ATD (Sigma) postnatally. Male neonates were treated with Silastic capsules containing ATD on PND 0 through 21. Procedures were the same as those used for the early postnatal TP and C treatments. This treatment was used because Experiment 1 attempted to determine if the experimental males would show sexual behavior with another male. However, during the sexual behavior tests when the males could interact, the stimulus males would often try to mount the experimental male (no difference seen between treatment groups). The experimental male would then react by turning on his back in order to reject the mount attempt but did not display further aggressive behaviors directed toward the stimulus male. In an attempt to reduce mount attempts by the stimulus males, ATD was administered to the stimulus males. Males treated postnatally with ATD have been shown to display lordosis when paired with another male (Bakker et al., 1996), and our ATD stimulus males showed statistically fewer attempted mounts than the untreated stimulus males of Experiment 1 (data not shown).
Animals were maintained in a facility accredited by the Association for Assessment and Accreditation of Laboratory Animal Care, and all experimental procedures were approved by the Michigan State University Animal Care and Use Committee.
Behavioral Testing (Experiments 1-3)
Partner Preference
Tests for partner preference (20-minute duration) were ran as described previously (Cummings et al., 2008; Henley et al., 2009). Briefly, one intact male and one sexually receptive female stimulus animal were tethered in the outer two chambers of a Plexiglas three-chamber apparatus (91 × 61 × 41 cm). The experimental male was able to move freely among the three chambers. Both experimental and stimulus animals were adapted to the apparatus, and stimulus animals were adapted to the harness prior to testing. Adaptations occurred twice for 30 min each prior to testing. All behavioral testing took place under dim red light illumination in the middle part of the dark phase of the light-dark cycle.
The tests were videotaped, and the following behaviors were quantified: time spent in each chamber, latency to first enter each chamber, and the occurrence of mounts, intromissions, and ejaculations shown by the experimental male towards the stimulus animals. Data from all behavioral tests were analyzed using The Observer 5.0 (Noldus), a behavioral data acquisition computer program. Preference scores were calculated by subtracting the amount of time spent in the stimulus male chamber from the amount of time spent in the stimulus female chamber. Therefore, a positive preference score indicates more time spent with the stimulus female, whereas a negative preference score indicates more time spent with the stimulus male. The experimental males were sexually naïve prior to the initial partner preference test.
Sexual behavior
Tests for sexual behavior displayed by the experimental males were conducted in a Plexiglas observation chamber (46 × 58 × 51 cm). During the test, the experimental male had unrestricted access to the stimulus animal (either male or female; see “Testing Schedule” section below for more details). The tests lasted 25 minutes or until ejaculation, whichever occurred first. Behavioral testing took place under dim red light illumination in the middle part of the dark phase of the light-dark cycle. Video recordings of these tests were analyzed to determine frequency of mounts, intromissions, and ejaculations shown by the experimental males and the latency to show these behaviors.
Testing Schedule
The initial partner preference of the male was tested in week 1. Each experimental male received sexual experience with both male and female stimulus animals during weeks 2 and 3, but data were not collected. During these experience conditions, experimental males were partnered with stimulus animals for 30 minutes during which time sexual behavior could occur. During week 4, half of the experimental males were tested for sexual behavior with a male and the other half with a female. The sex of the stimulus animals was reversed for week 5. Sexual behavior during weeks 4 and 5 was recorded and scored. In week 6, the male’s final partner preference was evaluated.
Hormone Assays (Experiments 1,2)
At least 5 months after behavioral tests were concluded, blood from deeply anesthetized (Nembutal, 75 mg/kg, intraperitoneal injection) experimental males was collected intracardially into heparinized tubes. The samples were kept on ice for 30 minutes, and then centrifuged at 8° C for 20 minutes at 3000 rcf. The supernatant was removed and stored at −20° C.
A solid-phase radioimmunoassay that uses 125iodine-labeled steroid, which competes with steroid in the sample for antibody sites in a polypropylene tube, was used to determine the amount of hormones in the samples. Testosterone and estradiol were measured using the Coat-A-Count Testosterone (Siemens) and Active Estradiol (Diagnostic Systems Laboratories, Inc.) radioimmunoassay kits, respectively. After incubation for 3 hours (testosterone) or 2 hours (estradiol) at 37° C, the tube was decanted and counted in a gamma counter. The quantity of hormone present in the sample was determined from a calibration curve. The analytical sensitivities for the assays were 4ng/dL (testosterone) and 11pg/ml (estradiol). Both assays show high precision and low to no crossreactivity to other compounds. Radioimmunoassays were completed by the Diagnostic Center for Population and Animal Health at Michigan State University. A randomly selected subset of males from both Experiment 1 and Experiment 2 were used for the radioimmunoassay (subset of males: Experiment 1, TP n=7, C n=6; Experiment 2, TP n=8, C n=6). For each treatment group, testosterone levels were not significantly different across experiments, and the samples from Experiment 1 and 2 were combined to test the effects of early exposure to exogenous testosterone.
Analysis
The data for the behavioral measures during the partner preference tests were analyzed using a 2 × 2 (perinatal treatment × initial or final test) ANOVA with repeated measures on the second factor. The data for the behavioral measures during the sexual behavior tests and for the hormone levels were analyzed using an independent samples t-test. A correlation coefficient (Pearson’s r) was used to examine whether serum testosterone or estradiol levels correlated with preference score.
For some variables, the data did not meet homogeneity of variance assumptions, even after the prescribed transformations (i.e. square root). For these measures, nonparametric statistics (Mann-Whitney U, Fisher’s Exact Probability, and Wilcoxon Signed Ranks test) were used for analysis.
RESULTS
Experiment 1 – Early Postnatal TP
Partner Preference
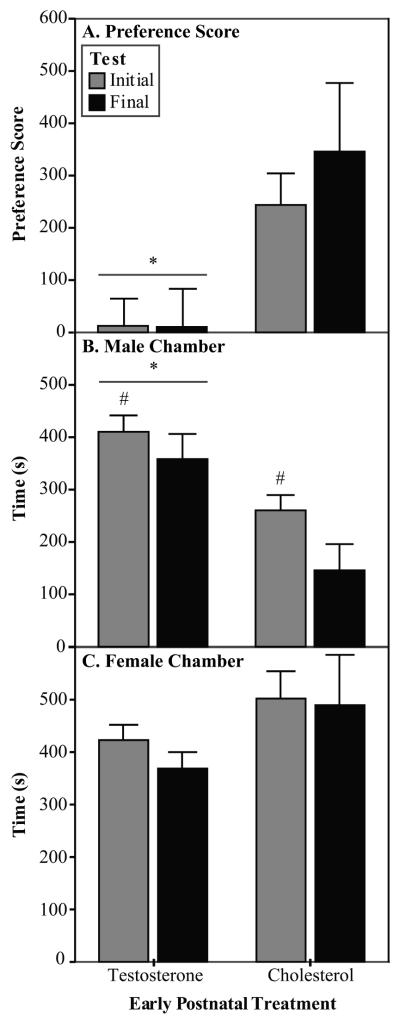
Early postnatal treatment with testosterone altered the partner preference of the experimental animals. The C males showed a higher preference score than that of the TP males (Fig. 1A; [F(1,27) = 10.9, p=0.003]). The change in preference score was a result of the TP males spending more time in the stimulus male chamber during both partner preference tests than did the C males (Fig. 1B; [F(1,27) = 18.0, p<0.001]), but testosterone treatment did not alter the amount of time spent in the female chamber (Fig. 1C).
Fig. 1.
Partner preference data (Experiment 1). In Experiment 1, the experimental males were exposed to early postnatal treatments and stimulus males were untreated. A) The C males showed a higher preference score than did the TP males in the partner preference tests. Preference score is calculated as time spent with stimulus female minus the time spent with stimulus male. B) The TP males spent more time in the stimulus male chamber than did the C males. C) The amount of time spent with the stimulus female was not affected by early postnatal treatment. TP n=17, C n=12 *Significantly different from C males. #Significantly different from Final test.
The latency to enter the stimulus female chamber and time spent with the male were affected by a main effect of week of test with no significant interaction. Regardless of early postnatal hormone treatment, the males decreased their latency to enter the female chamber from initial to final test (: all measures in seconds: 39.3 ± 4.2 vs. 23.2 ± 2.8; [F(1,27) = 12.7, p=0.001]). They also decreased the amount of time spent in the stimulus male chamber from initial to final test (Fig. 1B; [F(1,27) = 4.2, p=0.05]). In addition, TP males spent less time alone in the middle chamber during the initial partner preference test than during the final test (Wilcoxon signed ranks test; ± SEM: 366.8 ± 41.8 vs. 472.6 ± 47.6; p=0.03, df=16, Z=−2.15).
The proportion of males that showed sexual behavior with the stimulus female during the partner preference test was affected by early hormone treatment. More C males showed mounts and intromissions during the initial test, and more mounts, intromissions, and ejaculations during the final test compared with the TP males (Table 1; Fisher’s exact probability test). No significant group differences were seen in behaviors directed to the stimulus male.
Table 1.
Proportion of experimental males that displayed sexual behavior with the stimulus female during the partner preference and sexual behavior tests (Experiment 1). In Experiment 1, the experimental males were exposed to early postnatal treatments and stimulus males were untreated. C males were more likely to display male sexual behavior with the stimulus female than were TP males in both partner preference tests and the sexual behavior test during Experiment 1.
| Initial Partner Preference Test |
Final Partner Preference Test | Sexual Behavior Test | ||||||
|---|---|---|---|---|---|---|---|---|
| Behavior | Mount | Intromission | Mount | Intromission | Ejaculation | Mount | Intromission | Ejaculation |
| C | 6/12 | 4/12 | 10/14 | 10/14 | 8/14 | 11/13 | 11/13 | 9/13 |
| TP | 1/17* | 0/17* | 3/18* | 2/18* | 1/18* | 5/18** | 4/18** | 2/18** |
Significantly different from cholesterol, p<0.02
Significantly different from cholesterol, p<0.002
Sexual Behavior
Early postnatal testosterone treatment altered the expression of male sexual behavior when paired with a stimulus female. The proportion of males that exhibited sexual behaviors was analyzed in place of behavior frequency and latency since so few TP males displayed sexual behavior with the stimulus female. The proportion of males that showed sexual behaviors when paired with a female was higher in the C male group than in the TP male group (Table 1; Fisher’s exact probability test). When paired with a stimulus male, none of the behaviors scored were displayed in sufficient frequency to allow for statistical analysis.
Experiment 2 – Early Postnatal TP and ATD Stimulus Male
Partner Preference
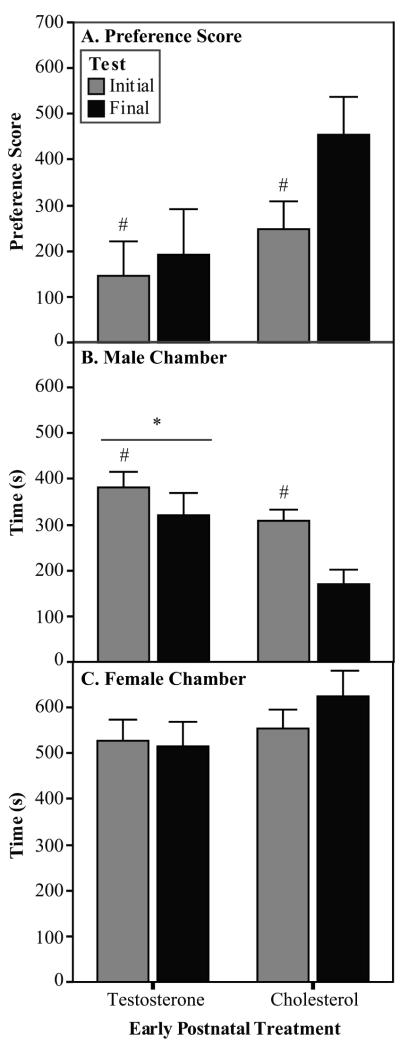
Treatment with testosterone early in development altered the partner preference of the male experimental animals. The TP males spent more time in the ATD stimulus male chamber during the partner preferences tests than did the C males (Fig. 2B; [F(1,31) = 4.1, p=0.05]; data subjected to a square root transformation). There was also a trend toward the C males showing a higher preference score than the TP males (Fig. 2A; [F(1,31) = 3.1, p<0.09]). Early hormone treatment did not affect the amount of time spent in the female chamber (Fig. 2C).
Fig. 2.
Partner preference data (Experiment 2). In Experiment 2, the experimental males were exposed to early postnatal treatments and stimulus males were treated with ATD. A) The C males showed a trend for a higher preference score than did the TP males in the partner preference tests. Preference score is calculated as time spent with stimulus female minus the time spent with stimulus male. B) The TP males spent more time in the stimulus male chamber than did the C males. C) The amount of time spent with the stimulus female was not affected by early postnatal treatment. TP n=17, C n=16 *Significantly different from C males. #Significantly different from Final test.
Sexual experience also altered the partner preference of the experimental males regardless of hormone treatment. Across both hormone treatments, the average latency to enter the female chamber ( ± SEM: 20.1 + 2.0 vs 55.5 + 6.4; [F(1,31) = 38.2, p<0.001]), and the latency to enter the ATD male chamber were shorter for the final test than for the initial test ( ± SEM: 29.3 + 3.4 vs 76.4 + 18.6; [F(1,31) = 5.8, p=0.02]). The experimental males spent less time in the middle chamber ( ± SEM: 311.1 + 21.3 vs 382.1 + 24.9; [F(1,31) = 5.6, p=0.02]) and more time in the ATD male chamber during the initial test compared with the final test (Fig. 3B; [F(1,31) = 14.7, p=0.001]). Finally, the males had a lower preference score during the initial test than during the final test (Fig. 3C; [F(1,31) = 6.4, p=0.02]).
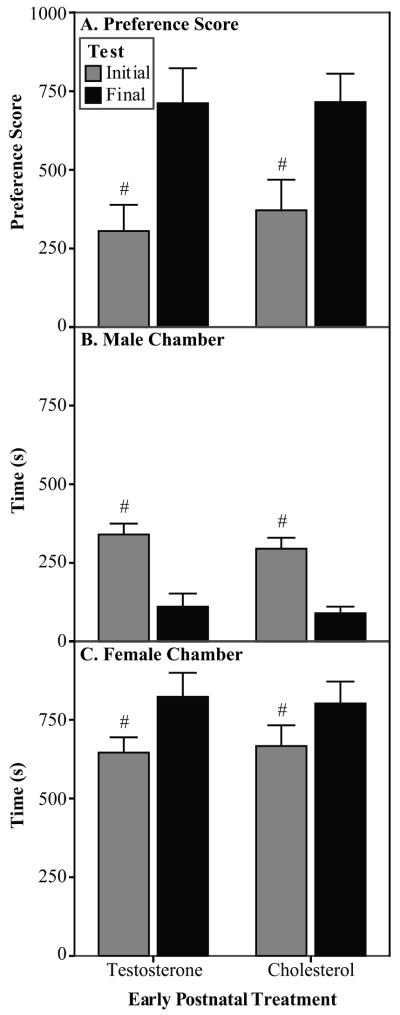
Fig. 3.
Partner preference data (Experiment 3). In Experiment 3, the experimental males were exposed to prenatal treatments and stimulus males were untreated. A) All males increased their preference score from initial to final test. Preference score is calculated as time spent with stimulus female minus the time spent with stimulus male. B) All males decreased the amount of time spent with the stimulus male from initial to final test. C) All males increased the amount of time spent with the stimulus female from initial to final test. TP n=7, C n=7 #Significantly different from Final test.
The proportion of males that showed sexual behavior with the stimulus female during the partner preference test was affected by early hormone treatment. More C males showed mounts, intromissions, and ejaculations during the final test compared with the TP males (Table 2; Fisher’s exact probability test). No differences were seen during the initial partner preference test or in behaviors shown with the stimulus male.
Table 2.
Proportion of experimental males that displayed sexual behavior with the stimulus female during the partner preference and sexual behavior tests (Experiment 2). In Experiment 2, experimental males were exposed to early postnatal treatments and stimulus males were treated with ATD. C males were more likely to display male sexual behavior with the stimulus female than were TP males in the final partner preference test and sexual behavior test during Experiment 2.
| Final Partner Preference Test | Sexual Behavior Test | |||||
|---|---|---|---|---|---|---|
| Behavior | Mount | Intromission | Ejaculation | Mount | Intromission | Ejaculation |
| C | 15/17 | 15/17 | 12/17 | 19/21 | 19/21 | 16/21 |
| TP | 2/18** | 2/18** | 1/18** | 6/20** | 6/20** | 5/20** |
Significantly different from cholesterol, p<0.002.
Sexual Behavior
Early postnatal testosterone treatment altered the expression of male sexual behavior when paired with a stimulus female. The proportion of males that exhibited sexual behaviors was analyzed in place of behavior frequency and latency since so few TP males displayed sexual behavior with the stimulus female. The proportion of males that showed sexual behaviors when paired with a female was higher in the C male group than in the TP male group (Table 2; Fisher’s exact probability test). When paired with a stimulus male, none of the behaviors scored were displayed in sufficient frequency to allow for statistical analysis.
Hormone Assays (Experiments 1,2)
The amount of circulating testosterone was affected by the early postnatal testosterone treatment during early life. The C males had significantly higher circulating testosterone levels than did the TP males ( ± SEM ng/ml: TP: 0.7 + 1.4 vs C: 3.5 + 3.7; t=−2.7, df=25, p=0.01). Circulating levels of estradiol, however, were not affected by the early hormone treatments ( ± SEM pg/ml: TP: 11.9 + 1.5 vs C: 12.2 + 2.9). To test the possibility that the reduction in circulating testosterone was directly responsible for the behavioral changes observed, a correlation was run between serum hormone levels and preference score. No significant correlation between preference score and testosterone level (r=0.1, p=0.61) or estradiol level (r=0.2, p=0.38) was seen.
Experiment 3 – Prenatal TP
Partner Preference and Sexual Behavior
Prenatal testosterone treatment did not have an effect on preference score (Fig. 3A), time spent with the male (Fig. 3B), or time spent with the female (Fig. 3C). However, sexual experience altered the partner preference of the experimental males regardless of hormone treatment. From initial to final test, the males showed an increased preference score (Fig. 3A; [F(1,12) = 24.8, p<0.001]), an increase in the time spent in the female chamber (Fig. 3C; [F(1,12) = 8.0, p=0.015]), and a decrease in the time spent in the male chamber (Fig. 3B; [F(1,12) = 75.8, p<0.001]). Finally, the latency to enter the female chamber was longer during the initial partner preference test compared to the final ( ± SEM: 51.0 ± 11.4 vs. 14.4 ± 3.1; [F(1,12) = 24.9, p<0.001]; data subjected to a square root transformation).
The latency to enter the stimulus male chamber was affected by a test by prenatal treatment interaction ([F(1,12) = 14.2, p=0.003]; data subjected to a square root transformation). During the initial test, the Oil males took longer to enter the male chamber than did the TP males ( ± SEM: 79.8 ± 13.5 vs. 23.3 ± 13.5). During the final test, the Oil males decreased their latency to enter the chamber compared to the initial test ( ± SEM: 79.8 ± 13.5 vs. 17.9 ± 6.3), but the TP males showed no change ( ± SEM: 23.3 ± 13.5 vs. 35.9 ± 6.3).
Prenatal testosterone treatment only had an effect on one sexual behavior measure during the partner preference tests. During the final test, the TP males showed fewer intromissions than did the Oil males (Mann-Whitney U; U=5.5; ± SEM: 6.3 ± 2.8 vs. 19.4 ± 2.8). No behaviors measured during the sexual behavior tests were affected by the prenatal testosterone treatment.
DISCUSSION
The overall finding of these studies was that male rats exposed to exogenous testosterone during early postnatal development showed altered partner preference and reduced sexual behavior as adults. Prenatal testosterone treatment did not affect these measures. These data do not support the hypothesis that the reduction in male sexual behavior seen after early hyper-androgen treatment is a result of a reduced motivation to approach the female. Postnatal testosterone treatment increased the time spent with a stimulus male, but did not alter for the time spent with a stimulus female. Under natural conditions, when competition among males is high, males exposed to supra-physiological levels of testosterone would be at a great disadvantage for successful matings. Not only do they show a lower incidence of sexual behavior with a female, but they would be as likely to approach a male as they would a female for a partner. Unexposed males, on the other hand, would be more likely to approach only females, thereby increasing their reproductive success. The results appear to support a role of high early androgen for the development of a bi-sexual social preference more so than a homosexual preference. If this effect also occurs in humans, it would be important to determine how such a preference interacts with environmental and cultural factors to influence sexual orientation.
Throughout this discussion we refer to a male’s choice of spending time with a male or female stimulus animal as “partner preference”. While this is clearly an operational definition, it should also be noted that the functional significance of this choice is unknown. Often partner preference is used in the context of “preference for a sexual partner”, but it is by no means clear that the choices made by the experimental males in this experiment were based on sexual motivation. It is equally likely that the choices emerged from some other type of social motivation such as affiliation. No agonistic behaviors were seen except for mount refusal, and most of the time, the animals were engaged in self-maintenance or resting behaviors. Until the appropriate functional studies are completed, it would seem prudent in this case to view “partner preference” simply as an operationally defined category of behavior.
Early Postnatal Hormone Effects
Regardless of the treatment of the stimulus male (Experiment 1: untreated; Experiment 2: ATD-treated), early postnatal testosterone treatment altered partner preference by increasing the amount of time males spent in the stimulus male chamber compared to that of control males without altering time spent with the stimulus female. The C males showed a preference for the stimulus female, whereas the TP males had a significantly decreased preference score (time with female minus time with male) in Experiment 1 and a trend for a decreased preference score in Experiment 2.
Although the alteration in preference score in Experiment 2 did not reach a significant level, it should be noted that the higher preference scores shown by the TP males in Experiment 2 (ATD stimulus males) compared to Experiment 1 (untreated stimulus males) was due to the experimental males spending more time with the stimulus female in Experiment 2. The amount of time spent in the male chamber was the same between the two experiments. Therefore, the overall effect of TP males showing increased time with the stimulus male without affecting time with the female was the same in both experiments, regardless of the condition of the stimulus male.
Endogenous perinatal testosterone exposure is responsible for both masculinization and defeminization of brain and behavior (see Introduction and Adkins-Regan, 1988; Bakker, 2003; Baum, 1979). The current study indicates that the TP males are completely defeminized, since they showed rejection behavior and not lordosis in response to attempted mounts by the stimulus males (data not shown). However, the TP males may not be fully masculinized. The increase in the amount of time the TP males spent with the stimulus male might reflect either a demasculinization or an increase in aggressiveness. The decrease in sexual behavior shown with a stimulus female suggests a behavioral demasculinization. This partial demasculinization may also account for the greater tendency of the TP males to approach the stimulus male while still choosing to spend time with the stimulus female, considering that normal females do spend time with other estrous females as well as sexually active males (Fang and Clemens, 1999). The data do not support an explanation based on an increase in aggression; aside from the rejection behavior of the experimental males when the stimulus males tried to mount them, there was no fighting between the experimental and stimulus males, ruling out the argument that the TP males were more aggressive.
The deficit seen in male behavior after early testosterone treatment may be a result of changes within the medial preoptic area (MPOA), a region known to play a role in both appetitive and consummatory aspects of male sexual behavior (McCarthy et al, 2009). Males have a higher density of dendritic spines in the MPOA than do females, and this sexual dimorphism can be seen as early as the day of birth (Amateau and McCarthy, 2002), and synaptic spine density is positively correlated with male mounting behavior (McCarthy et al, 2009). There is a possibility that the supra-physiological levels of testosterone during development altered the masculinization of this pathway, leading to fewer dendritic spines in the MPOA of TP males compared to C males. This could cause a decrease in both motivation to approach the female and expression of male sexual behavior.
Early postnatal TP treatment also disrupted male sexual behavior. In both Experiments 1 and 2 during the final partner preference test and during the sexual behavior tests, the C males were more likely to show copulatory behavior with a stimulus female than were the TP males. The disruption of male sexual behavior is in agreement with other studies that find deficits in adult sexual behavior after perinatal testosterone treatment of intact male rats (Diamond et al., 1973; Piacsek and Hostetter, 1984; Pollak and Sachs, 1975; Zadina et al., 1979), ferrets (Baum and Schretlen, 1975), and hamsters (Vomachka et al., 1981). Given the current finding, this reduction in sexual behavior does not appear to be due to a reduced motivation to approach the female. However, it is still possible that the failure to copulate with the female is due to diminished sexual stimulus value of the receptive female to the androgenized male.
Adult circulating testosterone levels were also affected by early postnatal hormone treatment in the present study. C males had higher levels of serum testosterone than did the TP males. Male hamsters (Vomachka et al., 1981) and ferrets (Baum and Schretlen, 1975) given TP early in development also show a decrease in adult serum testosterone levels, and numerous studies have linked neonatal estrogen treatment to decreased adult testosterone production (for review, Delbes et al., 2007). One hypothesis concerning the effect of neonatal steroid treatment on adult hormone levels is that early estrogen exposure may have a direct inhibitory effect on the enzymes that produce testosterone (Goyal et al., 2004; Goyal et al., 2003). Estradiol exposure either during gestation in vivo (Majdic et al., 1996) or to fetal rat leydig cells in vitro (Tsai-Morris et al., 1986) caused a decrease in the androgen synthesizing enzyme 17α-hydroxylase. Therefore, the effect of early testosterone on circulating testosterone levels in adulthood may be due to the action of estrogenic metabolites of testosterone. This change in adult circulating testosterone level was seen without any alteration of adult circulating estradiol level supporting the notion that the reduction in testosterone is not due to enhanced peripheral aromatization of testosterone to estradiol.
Although early postnatal treatment with TP reduced testosterone levels, we found no significant correlation between serum testosterone levels and preference scores. Plasma testosterone concentrations as low as 0.7 ng/ml, which was the mean concentration of serum testosterone seen in the early testosterone-treated animals of this study, maintain male copulatory behavior in castrated adult rats (Damassa et al., 1977). Circulating levels of testosterone have not been shown to be reliable predictors of male sexual behavior (Bakker et al., 1995; Portillo et al., 2006). Therefore, although with the available data a role for the low levels of testosterone cannot be ruled out, it is unlikely that the changes in partner preference seen after early postnatal treatment with TP are due to a reduction in serum testosterone. Also, no significant correlation was found between serum estradiol levels and preference score, indicating that the variations in the level of circulating hormones appears to play little role in the display of adult behavior.
Prenatal Hormone Effects
Only two measures were altered by prenatal testosterone treatment. During the initial test, the TP males entered the male chamber after a shorter latency than did the Oil males, and in the final partner preference test, the TP males achieved fewer intromissions than did the Oil males; no other sexual behavior measured during the preference tests was altered by the early hormone treatment. Therefore, prenatal TP treatment did not have a large effect on partner preference or sexual behavior.
Although normal levels of testosterone during the prenatal period have been shown to be necessary for the development of male-typical behaviors in adulthood (Clemens et al., 1978; Hoepfner and Ward, 1988; Neumann et al., 1966; Pollak and Sachs, 1975), it appears that supra-physiological levels of testosterone administered prenatally do not significantly alter male behavior, in contrast to the disruptive effects seen with early postnatal exposure. This outcome is similar to that seen after treatment with other agents, such as environmental contaminants that can affect steroid sensitive systems. Gestational treatment of a pregnant dam with polychlorinated biphenyls (PCBs), does not affect female offspring partner preference, whereas lactational exposure of female offspring to PCB significantly decreases the amount of time spent with a stimulus male (Cummings et al., 2008). In addition, early postnatal dioxin exposure increases the expression of female-like behaviors in adult male rats, but this alteration in behavior is not seen after prenatal exposure alone (Bjerke and Peterson, 1994). These observations identify the early postnatal period as a time during which the process of sexual differentiation is particularly sensitive to endogenous and environmental challenges.
Experience Effects
Regardless of perinatal hormone treatment, a number of behavioral measures changed significantly from the first to the second partner preference test in all experiments. From initial to final test, the experimental males showed a reduced amount of time spent with the stimulus male (Experiments 1, 2, 3), an increased preference score (Experiments 2, 3), an increase in time spent with the stimulus female (Experiment 3), and an increase in time spent in the middle chamber (Experiments 1, 2). Partner preference measures have been shown to be altered after receiving sexual experience. Specifically, sexual experience leads a male to display an increased preference for a female over a male stimulus animal (Matuszczyk and Larsson, 1994).
Latency to enter each chamber was originally evaluated as a measure of the experimental male’s motivation to approach each stimulus animal. In all three experiments, the males took less time to enter the female chamber during the final test compared to the initial. In Experiment 2, the males also took less time to enter the male chamber during the final test. The only treatment effect seen was in Experiment 3, following prenatal TP treatment. During the initial test, the TP males entered the male chamber sooner than the Oil males, but this difference was not seen in the final test, as the Oil males decreased their latency. The changes in latency may be due to the sexual experience received, or it could have been a result of the experimental males becoming more familiar with the testing apparatus and stimulus animals. However, since the control males in both Experiments 2 and 3 decreased their latency to enter the male chamber while also decreasing the amount of time spent with the male, in this study latency may not be a preferred indicator of motivation.
Summary
Early postnatal, but not prenatal, exogenous testosterone treatment disrupted male sexual behavior and altered the partner preference of intact males by increasing the time spent with a stimulus male without affecting the time spent with a stimulus female. It is unlikely that the reduction in male sexual behavior seen after early testosterone exposure is a result of reduced motivation to approach a female.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Adkins-Regan E. Sex hormones and sexual orientation in animals. Psychobiology. 1988;16(4):335–347. [Google Scholar]
- Bakker J. Sexual differentiation of the neuroendocrine mechanisms regulating mate recognition in mammals. Journal Of Neuroendocrinology. 2003;15(6):615–621. doi: 10.1046/j.1365-2826.2003.01036.x. [DOI] [PubMed] [Google Scholar]
- Bakker J, vanOphemert J, Slob AK. Sexual differentiation of odor and partner preference in the rat. Physiology & Behavior. 1996;60(2):489–494. doi: 10.1016/s0031-9384(96)80023-0. [DOI] [PubMed] [Google Scholar]
- Bakker J, Vanophemert J, Timmerman MA, Dejong FH, Slob AK. Endogenous reproductive hormones and nocturnal rhythms in partner preference and sexual behavior of ATD-treated male rats. Neuroendocrinology. 1995;62(4):396–405. doi: 10.1159/000127029. [DOI] [PubMed] [Google Scholar]
- Baum MJ. Differentiation Of Coital Behavior In Mammals - Comparative-Analysis. Neuroscience And Biobehavioral Reviews. 1979;3(4):265–284. doi: 10.1016/0149-7634(79)90013-7. [DOI] [PubMed] [Google Scholar]
- Baum MJ, Erskine MS, Kornberg E, Weaver CE. Prenatal And Neonatal Testosterone Exposure Interact To Affect Differentiation Of Sexual-Behavior And Partner Preference In Female Ferrets. Behavioral Neuroscience. 1990;104(1):183–198. doi: 10.1037//0735-7044.104.1.183. [DOI] [PubMed] [Google Scholar]
- Baum MJ, Schretlen P. Neuroendocrine effects of perinatal androgenization in the male ferret. Progress in Brain Reserach. 1975;42:343–55. doi: 10.1016/S0079-6123(08)63691-2. [DOI] [PubMed] [Google Scholar]
- Beach FA, Holz AM. Mating Behavior In Male Rats Castrated At Various Ages And Injected With Androgen. Journal Of Experimental Zoology. 1946;101(1):91–142. doi: 10.1002/jez.1401010107. [DOI] [PubMed] [Google Scholar]
- Beach FA, Noble RG, Orndoff RK. Effects Of Perinatal Androgen Treatment On Responses Of Male Rats To Gonadal Hormones In Adulthood. Journal Of Comparative And Physiological Psychology. 1969;68(4):490–497. doi: 10.1037/h0027658. [DOI] [PubMed] [Google Scholar]
- Berenbaum SA, Bryk KK, Nowak N, Quigley CA, Moffat S. Fingers as a Marker of Prenatal Androgen Exposure. Endocrinology. 2009;150(11):5119–5124. doi: 10.1210/en.2009-0774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bjerke DL, Peterson RE. Reproductive Toxicity Of 2,3,7,8-Tetrachlorodibenzo-P-Dioxin In Male-Rats - Different Effects Of In-Utero Versus Lactational Exposure. Toxicology And Applied Pharmacology. 1994;127(2):241–249. doi: 10.1006/taap.1994.1158. [DOI] [PubMed] [Google Scholar]
- Bogaert AF, Hershberger S. The relation between sexual orientation and penile size. Archives of Sexual Behavior. 1999;28(3):213–221. doi: 10.1023/a:1018780108597. [DOI] [PubMed] [Google Scholar]
- Clemens LG, Gladue BA, Coniglio LP. Prenatal Endogenous Androgenic Influences On Masculine Sexual-Behavior And Genital Morphology In Male And Female Rats. Hormones And Behavior. 1978;10(1):40–53. doi: 10.1016/0018-506x(78)90023-5. [DOI] [PubMed] [Google Scholar]
- Cummings JA, Clemens LG, Nunez AA. Exposure to PCB 77 affects partner preference but not sexual behavior in the female rat. Physiology & Behavior. 2008;95(3):471–475. doi: 10.1016/j.physbeh.2008.07.016. [DOI] [PubMed] [Google Scholar]
- Damassa DA, Smith ER, Tennent B, Davidson JM. Relationship Between Circulating Testosterone Levels And Male Sexual-Behavior In Rats. Hormones And Behavior. 1977;8(3):275–286. doi: 10.1016/0018-506x(77)90002-2. [DOI] [PubMed] [Google Scholar]
- Delbes G, Duquenne C, Szenker J, Taccoen J, Habert R, Levacher C. Developmental changes in testicular sensitivity to estrogens throughout fetal and neonatal life. Toxicological Sciences. 2007;99(1):234–243. doi: 10.1093/toxsci/kfm160. [DOI] [PubMed] [Google Scholar]
- Diamond M, Llacuna A, Wong CL. Sex Behavior After Neonatal Progesterone, Testosterone, Estrogen, Or Antiandrogens. Hormones And Behavior. 1973;4(1-2):73–88. [Google Scholar]
- Fang JM, Clemens LG. Contextual determinants of female-female mounting in laboratory rats. Animal Behaviour. 1999;57:545–555. doi: 10.1006/anbe.1998.1025. [DOI] [PubMed] [Google Scholar]
- Goyal HO, Braden TD, Williams CS, Dalvi P, Williams JW, Srivastava KK. Exposure of neonatal male rats to estrogen induces abnormal morphology of the penis and loss of fertility. Reproductive Toxicology. 2004;18(2):265–274. doi: 10.1016/j.reprotox.2003.12.001. [DOI] [PubMed] [Google Scholar]
- Goyal HO, Robateau A, Braden TD, Williams CS, Srivastava KK, Ali K. Neonatal estrogen exposure of male rats alters reproductive functions at adulthood. Biology Of Reproduction. 2003;68(6):2081–2091. doi: 10.1095/biolreprod.102.010637. [DOI] [PubMed] [Google Scholar]
- Henley CL, Nunez AA, Clemens LG. Estrogen treatment during development alters adult partner preference and reproductive behavior in female laboratory rats. Hormones and Behavior. 2009;55(1):68. doi: 10.1016/j.yhbeh.2008.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernandez-Tristan R, Cerezo AL. Antihormonal treatment during early development affects sexual function and behavior in male rats. Neuroscience Research Communications. 2000;26(2):129–138. [Google Scholar]
- Hoepfner BA, Ward IL. Prenatal And Neonatal Androgen Exposure Interact To Affect Sexual-Differentiation In Female Rats. Behavioral Neuroscience. 1988;102(1):61–65. doi: 10.1037//0735-7044.102.1.61. [DOI] [PubMed] [Google Scholar]
- Majdic G, Sharpe RM, Oshaughnessy PJ, Saunders PTK. Expression of cytochrome P450 17 alpha-hydroxylase/C17-20 lyase in the fetal rat testis is reduced by maternal exposure to exogenous estrogens. Endocrinology. 1996;137(3):1063–1070. doi: 10.1210/endo.137.3.8603575. [DOI] [PubMed] [Google Scholar]
- Matuszczyk JV, Larsson K. Experience Modulates The Influence Of Gonadal-Hormones On Sexual Orientation Of Male-Rats. Physiology & Behavior. 1994;55(3):527–531. doi: 10.1016/0031-9384(94)90112-0. [DOI] [PubMed] [Google Scholar]
- McFadden D, Champlin CA. Comparison of auditory evoked potentials in heterosexual, homosexual, and bisexual males and females. Jaro. 2000;1(1):89–99. doi: 10.1007/s101620010008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mullins RF, Levine S. Hormonal Determinants During Infancy Of Adult Sexual Behavior In Female Rat. Physiology & Behavior. 1968;3(2):333–338. [Google Scholar]
- Neumann F, Elger W, Kramer M. Development Of A Vagina In Male Rats By Inhibiting Androgen Receptors With An Anti-Androgen During Critical Phase Of Organogenesis. Endocrinology. 1966;78(3):628. doi: 10.1210/endo-78-3-628. &. [DOI] [PubMed] [Google Scholar]
- Piacsek BE, Hostetter MW. Neonatal Androgenization In The Male-Rat - Evidence For Central And Peripheral Defects. Biology Of Reproduction. 1984;30(2):344–351. doi: 10.1095/biolreprod30.2.344. [DOI] [PubMed] [Google Scholar]
- Pollak EI, Sachs BD. Masculine Sexual-Behavior And Morphology - Paradoxical Effects Of Perinatal Androgen Treatment In Male And Female Rats. Behavioral Biology. 1975;13(4):401–411. doi: 10.1016/s0091-6773(75)90949-9. [DOI] [PubMed] [Google Scholar]
- Portillo W, Diaz NF, Retana-Marquez S, Paredes RG. Olfactory, partner preference and Fos expression in the vomeronasal projection pathway of sexually sluggish male rats. Physiology & Behavior. 2006;88(4-5):389–397. doi: 10.1016/j.physbeh.2006.04.023. [DOI] [PubMed] [Google Scholar]
- Rahman Q. Fluctuating asymmetry, second to fourth finger length ratios and human sexual orientation. Psychoneuroendocrinology. 2005;30(4):382–391. doi: 10.1016/j.psyneuen.2004.10.006. [DOI] [PubMed] [Google Scholar]
- Rahman Q, Wilson GD. Sexual orientation and the 2nd to 4th finger length ratio: evidence for organising effects of sex hormones or developmental instability? Psychoneuroendocrinology. 2003;28(3):288–303. doi: 10.1016/s0306-4530(02)00022-7. [DOI] [PubMed] [Google Scholar]
- Robinson SJ, Manning JT. The ratio of 2nd to 4th digit length and male homosexuality. Evolution and Human Behavior. 2000;21(5):333–345. doi: 10.1016/s1090-5138(00)00052-0. [DOI] [PubMed] [Google Scholar]
- Tsai-Morris CH, Knox G, Luna S, Dufau ML. Acquisition Of Estradiol-Mediated Regulatory Mechanism Of Steroidogenesis In Cultured Fetal-Rat Leydig-Cells. Journal Of Biological Chemistry. 1986;261(8):3471–3474. [PubMed] [Google Scholar]
- Vomachka AJ, Ruppert PH, Greenwald GS, Clemens LG. Adult Sexual-Behavior Deficits And Altered Hormone Levels In Male Hamsters Given Steroids During Development. Physiology & Behavior. 1981;26(3):461–466. doi: 10.1016/0031-9384(81)90174-8. [DOI] [PubMed] [Google Scholar]
- Ward IL. Differential effect of pre- and postnatal androgen on the sexual behavior of intact and spayed female rats. Hormones and Behavior. 1969;1:25–36. [Google Scholar]
- Ward IL, Ward OB, Affuso JD, Long WD, French JA, Hendricks SE. Fetal testosterone surge: specific modulations induced in male rats by maternal stress and/or alcohol consumption. Hormones And Behavior. 2003;43(5):531–539. doi: 10.1016/s0018-506x(03)00061-8. [DOI] [PubMed] [Google Scholar]
- Whalen RE, Edwards DA. Hormonal determinants of the development of masculine and feminine behavior in male and female rats. The Anatomical Record. 1967;157(2):173–180. doi: 10.1002/ar.1091570208. [DOI] [PubMed] [Google Scholar]
- Zadina JE, Dunlap JL, Gerall AA. Modifications Induced By Neonatal Steroids In Reproductive-Organs And Behavior Of Male-Rats. Journal Of Comparative And Physiological Psychology. 1979;93(2):314–322. doi: 10.1037/h0077564. [DOI] [PubMed] [Google Scholar]