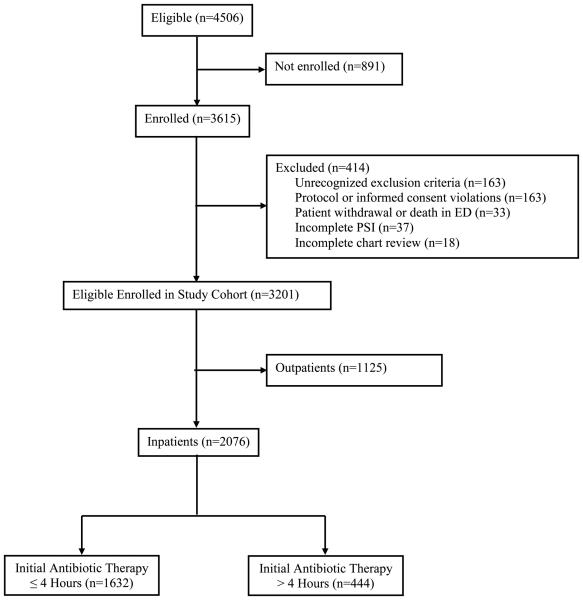
Figure 1. Patient Enrollment for the EDCAP Trial and Resulting Guideline Compliance with Timely Antibiotic Administration.
In the EDCAP Trial, a total of 4506 patients were eligible, 3615 were initially enrolled, and 414 were subsequently excluded. Reasons for exclusion included: (1) discovery of one or more exclusion criteria not recognized at presentation (n=163); (2) enrollment protocol or informed consent violations (n=163); (3) patient withdrawal or death in the emergency department before assignment of initial site of treatment (n=33); (4) incomplete information for PSI risk class (n=37); and (5) incomplete medical record review (n=18)., The most common reasons for protocol violations were: (1) enrollment of patients managed by a study investigator (n=47); (2) previous study enrollment (n=32); and (3) participation in a competing research protocol (n=29). Overall, of the 3201 patients who comprised the entire study cohort to assess initial processes of care, 2076 were treated as inpatients, of whom 1632 (78.6%) received initial antibiotic therapy within 4 hours of presentation.